With over 20 years of experience, the Clinical Trials Unit (CTU) has an international reputation for conducting clinical trials in a wide range of areas.
We have full accreditation from the UK Clinical Research Collaboration (UKCRC).
Our portfolio includes trials of drugs and complex interventions, mechanisms of disease and treatments, cohort studies and systematic reviews of evidence.
The LSHTM Clinical Trials Unit (CTU) is based within the largest school of public health in Europe with a global network of collaborators from over 70 countries.
The CTU has an international reputation for its clinical trials, especially in the areas of trauma and emergency care, cardiovascular disease, maternal health and complex environmental, social and behaviour change interventions.
We value working with patients and the public and they are at the centre of our research activities.
The CTU is accredited by the UK Clinical Research Collaboration.
Our research portfolio includes:
- trials of drugs
- complex interventions
- mechanisms of disease and treatments
- cohort studies and systematic reviews of evidence
We aim to improve the efficiency of trials to enhance quality, limit cost and reduce our carbon footprint. We do this by examining our trial designs, participant recruitment and retention strategies, and by monitoring how we conduct trials.
As well as running our own trials, we offer support and advice to colleagues and external parties planning clinical trials through our trial adoption process.
Who we work with
We work closely with funders and research partners around the world. We also collaborate with LSHTM research centres and teams.
Our partners
Within LSHTM we liaise closely with the:
- MRC The Gambia Clinical Trials Unit
- MRC Uganda Clinical Studies Support Section.
- Centre for Data and Statistical Science for Health
- Centre for Evaluation.
Analysis of Clinical Trials is one of the core themes of the Centre for Data and Statistical Science for Health. The Centre for Evaluation’s Quantifying Impact theme brings together specialists in the design, conduct, analysis, and reporting of evaluations that assess and quantify the impact of complex public health interventions, policies or health system changes. Its sub-themes are: cluster-randomised trials, stepped wedge trials, non-randomised and quasi experimental trials and pragmatic trials.
National co-ordinating centres
We have long-standing partnerships with research institutions in Nigeria, Pakistan and Zambia that act as national coordinating centres for many of our trauma and maternal health trials:
- College of Medicine, University of Ibadan, Nigeria
- Shifa Tameer-e-Millat University, Pakistan
- University Teaching Hospitals, Lusaka, Zambia
Meet the team
Our expert team includes clinical trialists, methodologists, statisticians, trial managers and administrators, data managers and IT systems and software development experts.
Our approach
- Identifying cost-effective interventions for problems of global health importance including a range of clinical trials in a number of research themes;
- Promoting the efficient conduct of clinical trials through statistical data monitoring of trials, developing in-house IT and database systems (including central randomisation, secure servers, trials databases and adverse event reporting systems);
- Maximising the use of clinical trial data by making anonymised datasets available to investigators beyond the original research team, within and external to LSHTM;
- Maximising the impact of clinical trials through prognosis research to improve clinical decision making, counsel patients and to facilitate clinical research;
- Increasing the health impact of clinical trials through more effective dissemination of trial results;
- Achieving more effective engagement in the design, conduct and dissemination of trial by increasing the emphasis on patient and public involvement in our trials.
Alex Lyons
Data manager

Andrew Thayne
Assistant Data Manager

Catherine Gilliam
Trial Administrator

Christiana Alao
Data assistant

Collette Barrow
Trial Administrator

Danielle Beaumont
Senior Trial Manager / Research Fellow

Danielle Prowse
Data Manager
Giada Susannini
Assistant Trial Manager
Ingrid Ker
Data Assistant
Kiran Bal
Assistant Trial Manager
Lily Nicholson
Assistant Trial Manager

Mbwana Mohamed
CTU Systems and Infrastructure Officer
Musa Faal
CTU Coordinator

Myriam Benyahia
Project Coordinator
Ruben Martin
Project Administrator
Current trials
- Cardiovascular
The CTU cardiovascular team specialises in the design, conduct and analysis of pragmatic multicentre clinical trials, often with simple interventions or treatment strategies.
We have developed strong links with many of the top cardiovascular hospitals in the UK over the past 30 years, beginning with the BHF-funded RITA, RITA-2 and RITA-3 trials. Through these collaborations with sites we aim to develop efficient trials which can be conducted with minimal complexity while delivering important results.
As well as trial design, conduct and management we have world renowned expertise in statistical methodology, analysis and interpretation of large scale cardiovascular trials. This includes development of novel statistical methods (such as the Win Ratio) and publication of educational articles specifically in the design and analysis of cardiovascular trials.
We also support the statistical design, data monitoring, analysis and publication for a larger set of major industry sponsored international cardiovascular trials.
We have direct experience in delivering emergency research in the NHS, for example in advance of primary percutaneous coronary intervention (PCI) (ERIC-PPCI), in cardiac arrest (ARREST), or within primary care (StatinWISE). We have worked on trials in coronary artery bypass surgery (ERICCA, RITA, Tight K), remote ischaemic pre-conditioning (ERICCA, ERIC-PPCI, REPAIR), percutaneous coronary Intervention (RITA, RITA-2, RITA-3, REVIVED, ERIC-PPCI, CHIP-BCIS3) and transcatheter aortic valve implantation (BHF PROTECT-TAVI).
ARREST
ARREST investigates expedited transfer to a specialist cardiac arrest centre in patients with non-ST elevation out-of-hospital cardiac arrest.
BHF PROTECT-TAVI
BHF PROTECT-TAVI is investigating the effect of Cerebral Embolic Protection on stroke after transcatheter aortic valve implantation (TAVI).
CHIP-BCIS3
CHIP-BCIS3 is investigating the role of left ventricular unloading in high risk angioplasty (stenting).
Tight K
Tight K is investigating whether maintenance of high-normal serum potassium levels in the immediate period after heart surgery is necessary to reduce arrhythmia.
- Emergency care
CRASH-4
CRASH-4 aims to provide reliable evidence about the effects of early intramuscular tranexamic acid (TXA) on intracranial haemorrhage, disability, death, and dementia in older adults with symptomatic mild head injury.
- Environmental, social, behaviour change
CHANGE
The CHANGE trial is testing the effectiveness of a contextually relevant transdiagnostic intervention addressing alcohol misuse with co-existing mental health conditions (depression, anxiety and post-traumatic stress disorders) among conflict-affected populations in Uganda and Ukraine.
Child-friendly Catholic Schools Study in Zimbabwe
(CCSS-Z)The Safe Schools Study is a whole-school behaviour change intervention conducted in Catholic primary schools in Zimbabwe, aiming to improve school climate, reduce teacher corporal punishment and peer bullying. Between 2024-2025 the intervention will be evaluated in a mixed-methods cluster randomised controlled trial.
ICAP
The Integrated Care for Alcohol Problems (ICAP) programme in India will develop and test the effectiveness of an integrated intervention for the person with drinking problems and their family members across the whole spectrum of alcohol use disorders.
MAMI
A RCT to test the Management of small and nutritionally At-risk Infants and their Mothers (MAMI) care pathway as a community-based approach to care for nutritionally at risk infants under six months within existing services in two sites in Ethiopia.
Pilot Trial of Good School Toolkit for secondary schools
The Good school Toolkit secondary pilot trial is a mixed-methods phase 2 pilot trial of a whole-school violence prevention intervention in Ugandan secondary schools. Following the encouraging results of the Good Schools Study in primary schools, this pilot trial aims to refine and finalise an adapted version of the Good School Toolkit for secondary schools, understand feasibility and delivery of the intervention, and explore design parameters for a subsequent phase 3 trial.
Positive Choices
Phase III RCT of Positive Choices: a whole-school social-marketing intervention to promote sexual health and reduce health inequalities.
Taking the Good School Toolkit to Scale
This mixed-methods study aims to determine the feasibility and acceptability of a new, scalable delivery model of the Good School Toolkit in primary and secondary schools in Uganda. In this new model, schools are supported by Regional Resource Persons, who are community facilitators trained by Raising Voices to support schools close to their home districts to implement the toolkit.
- Maternal, sexual and reproductive health
I'M WOMAN
I’M WOMAN aims to assess the effects of intramuscular (IM) and intravenous tranexamic acid in women at increased risk of postpartum haemorrhage (PPH).
Supporting MumS
The Supporting MumS trial is a UK-wide, multi-centre randomised controlled trial that aims to examine the effectiveness of an automated text message intervention to support weight management in the postpartum period.
WOMAN trials
Programme of work into TXA for PPH (encompasses WOMAN, WOMAN-2, I'M WOMAN, WOMAN Pharmaco-TXA).
WOMAN2
Tranexamic acid for the prevention of postpartum bleeding in women with anaemia: an international, randomised, double-blind, placebo-controlled trial.
Completed trials
- Cardiovascular
AIMS
A prospective, randomised, placebo-controlled double blind, multi-centre study investigating the effects of irbesartan on aortic dilation in people with Marfan syndrome.
COPIA (feasibility study)
Comparison between Propofol and Inhaled Anaesthesia on cardiovascular morbidity and mortality following cardiac surgery, a multicentre randomised controlled trial.
ERICCA
ERICCA investigated the effects of Remote Ischaemic Preconditioning (RIC) before heart bypass surgery.
ERIC-PPCI
ERIC-PPCI investigated the effects of Remote Ischaemic Preconditioning (RIC) in the acute period after ST-elevation myocardial infarction.
REVIVED-BCIS-2
REVIVED-BCIS-2 investigated the effectiveness of angioplasty (stenting) in patients living with heart failure.
StatinWISE
StatinWISE investigated whether the muscle symptoms experienced during statin use were caused by statins.
- Emergency care
BRAIN Trial
The BRAIN TRIAL was a randomised, placebo-controlled trial of a Bradykinin B2 receptor antagonist (Anatibant) in patients with traumatic brain injury. Funding was withdrawn by the sponsor during the trial.
CRASH
The corticosteroid randomisation after significant head injury (CRASH) trial showed that prognostic models can be used to obtain valid predictions of relevant outcomes in patients with traumatic brain injury.
CRASH-2
Results showed that tranexamic acid safely reduced the risk of death in bleeding trauma patients.
CRASH-3
The CRASH-3 trial provided the first evidence of a drug which can prevent death following Traumatic Brain Injury (TBI).
HALT-IT
The HALT-IT trial assessed whether administration of tranexamic acid in people with acute gastrointestinal bleeding can reduce their risk of dying in hospital.
Trauma-INTACT
Trauma-INTACT results show that tranexamic acid is well tolerated and rapidly absorbed via the intra-muscular route in bleeding trauma patients.
- Environmental, social, behaviour change
Learning Together
Pilot: Initiating change locally in bullying and aggression through the school environment. (INCLUSIVE): a randomised controlled trial
Full trial: Effects of the Learning Together intervention on bullying and aggression in English secondary schools (INCLUSIVE): a cluster randomised controlled trial.
Preventing Violence Against Children in Schools (PVACS)
The PVACS mixed-methods cluster randomised controlled trial evaluated the effect of the EmpaTeach intervention which aimed to train teachers on emotional self-regulation and positive discipline to prevent and reduce violence against children in schools in Nyarugusu refugee camp.
PREMIUM
The PREMIUM trial implemented evidence-based facility and community interventions to reduce the treatment gap for depression. The trial demonstrated the effectiveness of Counselling for Alcohol Problems (CAP), a lay counsellor delivered psychosocial intervention for harmful drinking in India.
Project Respect
Pilot: A school intervention for 13- to 15-year-olds to prevent dating and relationship violence: a cluster randomised controlled trial.
The Good Schools Study
The Good School Toolkit, developed by Ugandan NGO Raising Voices, is a whole-school intervention aiming to reduce violence against children in schools. A cluster randomised controlled trial of the intervention in Ugandan primary schools in 2014 showed that the Toolkit reduced past week physical violence from school staff to students by 42%, and improved students’ feelings of wellbeing and connection to schools.
For more details read these publications.
- Information technology
Internet-accessed sexually transmitted infection (e-STI) testing
A randomised, single-blind, controlled trial to assess the effectiveness of an e-STI testing and results service (chlamydia, gonorrhoea, HIV, and syphilis) on STI testing uptake and STI cases diagnosed.
SAFE TXT
A randomised controlled trial of an intervention delivered by mobile phone messaging to reduce STIs by increasing sexual health precaution behaviours in young people.
txt2stop
A single-blind, randomised trial assessing the effect of an automated smoking cessation programme delivered via mobile phone text messaging.
Intervention delivered by mobile phone to increase acceptability of effective contraception in Palestine, Tajikistan and Bolivia
Read about the trials results in Palestine: A randomized controlled trial of an intervention delivered by mobile phone text message to increase the acceptability of effective contraception among young women in Palestine
- Maternal, sexual and reproductive health
Pharmaco - TXA Trial
A phase 1 trial in healthy volunteers aimed at determining the bioavailability and pharmacokinetics of tranexamic acid following administration by different routes and relevant doses.
Read the publication: Physiologically based modelling of tranexamic acid pharmacokinetics following intravenous, intramuscular, subcutaneous, and oral administration in healthy volunteers
WOMAN-PharmacoTXA
WOMAN-PharmacoTXA was a randomised trial and pharmacological study in caesarean section births that investigated alternative routes for tranexamic acid (TXA) treatment in obstetric bleeding. More details are available in this video.
The trial showed that intramuscular administration of TXA is safe and quickly reaches therapeutic concentrations in pregnant women and may be a suitable alternative to intravenous TXA.
WOMAN Trial
Tranexamic acid for the treatment of postpartum haemorrhage: An international, randomised, double blind, placebo-controlled trial. The WOMAN trial showed that if given within three hours, tranexamic acid can reduce the risk of death due to bleeding by one third.
Sub-studies:
ETAPLaT: Effects of tranexamic acid on platelet function and thrombin generation
WOMAN-ETAC: Effect of tranexamic acid on coagulation and fibrinolysis in women with postpartum haemorrhage
- Other
Alleviating Hidden Hunger with Agronomy trial (AHHA)
The AHHA trial was an individually randomised placebo-controlled trial conducted in a rural setting in Malawi. The trial showed that consumption of maize flour biofortified using selenium fertilisers, was effective at improving selenium status of adult women and children following an 8-week intervention period.
PREVENTT
A randomised double-blind controlled phase III study to compare the efficacy and safety of intravenous ferric carboxymaltose with placebo in patients with anaemia undergoing major open abdominal surgery.
REPAIR
The REnal Protection Against Ischaemia-Reperfusion in transplantation (REPAIR) a multicentre, multinational, double-blind, factorial designed randomised controlled trial examined whether remote ischaemic preconditioning (RIPC) improved renal function after living-donor kidney transplantation.
We are fully accredited as a UK Clinical Research Collaboration (UKCRC) Clinical Trials Unit and offer support for clinical trial design, planning, implementation and analysis through our trial adoption process. We adopt trials led from within and outside LSHTM.
Trial adoption process
Enquire
- Submit an enquiry using our CTU collaboration enquiry form
- Most enquiries will already have a provisional proposal and a funding source in mind.
- All proposals must plan to include a member of the CTU management group as a co-applicant to be considered for adoption.
Outline review
- We will respond to your enquiry within 10 working days.
- If we can progress your enquiry, we will provide preliminary feedback and arrange to discuss it with you.
Full review
- A member of our team will work with you to develop your proposal. This will include costing for the role that the CTU will play if the application is successful.
- A well-developed draft proposal will undergo full review by an independent member of the CTU management group.
- Successful review results in the trial being ‘CTU Adopted’.
When reviewing proposals, we look for trials that satisfy our review criteria:
- Review criteria
Necessary
The evidence-base (including a formal review of relevant Randomised Controlled Trials) clearly demonstrates that the research is needed.
Important
The trial addresses a question that is clinically important, such as areas of crucial evidence gaps, or areas of controversy or high variation in clinical care.
Relevant
The trial is relevant to the study population.
Feasible
To ensure trials progress in line with expectations, in particular participant recruitment and retention, there is an appropriate blend of experience and clinical credibility in the research team.
The assumptions behind the study stand up to critical review, and evidence is provided that these assumptions are reasonable.
If no such reassurance is possible, a pilot study or early returns as the study commences will be needed.
Methodologically sound
The study is optimally designed, with appropriate analysis, suitable methods of managing and conducting the trial, the trial will be registered and there is full commitment to publish results in peer-reviewed journals.
Financially viable
Staffing and consumable costs are adequately funded and deliverable in the proposed timelines. The CTU involvement is appropriately financed.
Aligned
The study is aligned with the CTU’s areas of clinical trial excellence.
Ethically sound
High ethical standards are evident, and study risks and benefits have been properly considered.
Although some trials may be successful on all of the above criteria, if at the point of application the CTU does not have capacity to take on the necessary responsibilities, we will not adopt the trial.
Adoption
- Once a trial has been adopted, the CTU commits to its responsibilities as detailed in the proposal and related documentation.
- The progress of all CTU adopted trials is reviewed at regular intervals by the CTU management group, with particular attention paid to recruitment, retention and any serious adverse effects (SAEs), deviations or major risks identified.
- Management group
- Amy Brenner
- Cari Free
- Charles Opondo
- Danielle Beaumont
- Danielle Prowse
- Diana Elbourne
- Elizabeth Allen
- Jo Sturgess
- Linda Sharples
- Nicholas Connor
- Pa Modou Cham
- Richard Evans
- Shirine Voller
- Steven Robertson
- Tansy Edwards
- Tim Clayton
- Research methods, trial design and analysis
Our team of epidemiologists, systematic reviewers and trialists have expertise in the design, conduct, analysis and reporting of international randomised controlled trials across a range of subject areas and methodologies. We have experience in designing and implementing trials in a wide range of socio-economic settings and geographies. Our expertise includes:
- systematic review methodology
- clinical trial design
- methodological issues in clinical trials
- scientific writing
- effective dissemination of research findings.
- Trials and statistical methodology, monitoring and health economics
Our statisticians, trialists and health economists guide the design, feasibility, conduct, analysis and reporting of studies and we have strong links with the Centre for Statistical Methodology.
Our team of statisticians and health economists are specialists in:
- statistical analyses
- statistical monitoring
- health economic evaluation
- cost-effectiveness modelling
- report writing and publication
- dissemination of trial results.
We have expertise in the development of statistical and trials methodology, analysis and interpretation of large-scale trials as well as providing educational resources for the explanation and implementation of these key concepts in clinical trials. We have developed innovative and efficient methods for improving trial recruitment and ensuring high follow up. This methodological and educational work is undertaken to directly improve current and future clinical trials. For example, the development of the Win Ratio has led to this being adopted widely within the cardiovascular community including as the primary outcome for a number of major trials.
We are also skilled in using statistical methods to monitor trial data as it is collected. This is an efficient and cost-effective way to ensure data quality, patient safety and trial integrity. Statistical methods are used to highlight errors and omissions, detect outliers, check for low variance to identify unusual correlations that may suggest scientific misconduct or improper data collection. By identifying which trial sites require on-site monitoring and source data verification, support can be focused where it is most needed, reducing the environmental impact and cost of travel.
- Trial management
Our trial management teams have knowledge of every stage of the clinical trial life cycle from setup, planning and implementation through to reporting.
Expert trial managers with the training and experience to overcome operational challenges and technical complexities are often the difference between the success and failure of a clinical trial. Our professional trial staff contribute to UK Clinical Research Collaboration working groups and other networks to advance clinical trial management practice.
Our trial management expertise includes:
- protocol development
- funding applications
- setup of oversight bodies
- ethics committee, regulatory and NHS Trust research and development applications
- trial management and coordination
- good clinical practice (GCP) training
- participant recruitment
- quality control procedures
- case report forms (CRFs)
- database design, development and management
- data management
- design and management of trial websites.
- Data management and IT
Our data managers provide the expertise, services and infrastructure to assist with data management needs throughout a trial, from design and setup through to data review and analysis. We have experience of data management for small, single-site studies through to large-scale, multi-centre randomised trials with up to 30,000 participants. Our data managers are experienced in using Electronic Data Capture Systems such as REDCap and in the design and validation of bespoke systems.
We also have IT systems infrastructure and software development experts who design and maintain our databases and IT infrastructure and ensure that we meet the highest regulatory standards for data privacy and security.
Our data management and IT specialists are experts in:
- case report form (CRF) development and design
- creation of Data Management Plans and Standard Operating Procedures
- advice on data collection methods and data flow, including secure transmission of data
- database design and specifications; including for the development of data capture, randomisation and unblinding systems
- ensuring a high quality of computer systems validation to deliver a fully regulatory compliant database
- data review and cleaning
- management of pharmacovigilance data
- reporting for interim and final data analyses
- database development
- IT systems infrastructure oversight.
- Patient and Public Involvement
Patients and the public are at the centre of our work and are involved at every stage in the research process, from development of research ideas to communication of trial results.
The involvement of patients and the public in the early stages of trial development, to identify questions and outcomes important and relevant to them, is a key part of our approach to Patient and Public Involvement (PPI). We involve PPI in designing robust and relevant clinical trials, as co-applicants on the grants.
We include PPI representatives on management group and trial steering committees and include them in decision- making about the conduct of trials and the trial protocol, ensuring that a patient and public voice is heard throughout the timeline of our trials.
We are committed to enrolling a representative and diverse population, and high-quality PPI work has been and will continue to be central to our work to achieve that goal.
We involve patients and the public in the writing and dissemination of trial results, both to clinicians and to the public.
- Equity and inclusion
We have developed and evaluated new effective methods to increase recruitment and follow up. We use approaches to ensure trial populations reflect the gender, social and ethnic diversity of populations they are recruited from, so there is equal opportunity to benefit from research. When we develop interventions, we do so with input from diverse social and ethnic groups to ensure interventions are relevant and acceptable to those with greatest health need.
- Environmental impact
Clinical trials are energy intensive and produce substantial greenhouse gas emissions. We need to establish awareness about the broader consequences of health care and research, and through awareness increase the pace of action to mitigate one of the most important global challenges of our time. This remains an area of interest to our CTU and to LSHTM more widely.
Clinical trials courses
LSHTM offers several clinical trials-related training courses.
MSc Clinical Trials
This programme provides students with a theoretical and practical understanding of the issues involved in the design, conduct, analysis and interpretation of randomised controlled trials of health interventions. It is suitable for students working in low-, middle- and high-income countries.
Short Course in Clinical Trials
The Essentials of Clinical Trials short course gives attendees a clear understanding of the fundamental principles of Randomised Clinical Trials (RCTs). The course is offered in a hybrid format with students choosing to attend in person in London or online.
Good Clinical Practice Training
LSHTM offers a free Good Clinical Practice training course. This covers the ethical and scientific quality requirements that must be followed when designing, conducting, recording and reporting clinical trials that involve people.
The tranexamic acid (TXA) trials, run by the Clinical Trials Unit at the London School of Hygiene & Tropical Medicine, have featured in the CaSE July briefing. CaSE, the Campaign for Science and Engineering, is the UK’s leading independent advocate for science and engineering. The TXA case study highlights two large scale trials looking at TXA for trauma and maternal health and the global impacts of these trials, highlighting the crucial role that universities play in the research and development ecosystem.
Explore the briefing to find out more
Evidence from the OMWaNA trial shows that kangaroo mother care (KMC) initiated before clinical stabilisation did not reduce early neonatal mortality but had other clinical benefits and was cost effective relative to incubator care. Findings from OMWaNA are published in the Lancet
The OMWaNA trial recruited 2,221 neonates across five hospital in Uganda between 2019 and 2023, aiming to assess the effectiveness, safety, costs and cost effectiveness of KMC initiated before clinical stabilisation when compared to standard care in neonates weighing up to 2000g. The study was led by researchers from the London School of Hygiene and Tropical Medicine and the Medical Research Council/Uganda Virus Research Institute (MRC/UKVI)
You can view the global webinar held to coincide with the publication of results, featuring presentations by the trial team, an interview with a trial participant, and a panel discussion with global health experts and policy makers here, and a blog describing experiences from the trial can be found here.
In an expert opinion piece; Richard Evans, Senior Manager of the LSHTM Clinical Trials Unit discusses the need to improve the representation of women at all stages in the design and implementation of cardiovascular trials. This builds on a recently published article in Nature Reviews Cardiology.
Women are underrepresented at all levels in cardiovascular trials including participants, clinicians and researchers. This leads to, and results from, inequities in healthcare with cardiovascular disease being underdiagnosed and undertreated in women. Members of the cardiovascular trials team at the London-based LSHTM Clinical Trials Unit have contributed to an article recently published in Nature Reviews Cardiology. The article sets out steps for addressing sex-based inequities in cardiovascular clinical trials. Read more about the work here.
The Medical Statistics Department (MSD) at the London School of Hygiene and Tropical Medicine (LSHTM) will be running a short course on the essentials of clinical trials from 1-5 July 2024. The course is relevant to those who are keen to gain an understanding of the rigorous evaluation of interventions in health care, including clinical research professionals, research managers, and other scientists with an interest in clinical trials. The course will be delivered using a hybrid format with students able to attend either in person at LSHTM or online. To find out more information and apply, please visit the LSHTM webpage.
The Directors of the London-based LSHTM Clinical Trials Unit (CTU) were hosted by colleagues at MRC/UVRI & LSHTM Uganda Research Unit from 6th-9th February 2024. The purpose of the visit was to strengthen collaboration on randomized controlled trials. It came at an opportune time as the Uganda unit is in the process of consolidating and extending its clinical trials capability under a new Clinical Trials and Research Platform. Read more about the visit on the Trials Network webpages
The Trials Network is running a series of six seminars through 2024 on Practical Issues in Clinical Trials. The seminars are open to all and will be run as online webinars.
The seminar series will feature speakers from across LSHTM and our research partners. Each seminar will include a brief overview of current practice, theory and evidence, followed by worked examples from different presenters.
- Seminar 1: Practical Issues in Clinical Trials seminar 1: PPIE and Community Involvement, Tuesday 6 February 1-2 pm GMT (Zoom webinar)
In the first seminar, Professor Caroline Free, co-director of the LSHTM Clinical Trials Unit, Dr Armel Zemsi, Head of the Clinical Trials Unit at the MRC Unit The Gambia at LSHTM and colleagues from the MRC/UVRI & LSHTM Uganda Research Unit will present on the state of current evidence and practice around patient and community involvement in research, and share concrete examples from their respective regions in order to provide an overview on what works and the lessons learned.
Future seminars in the series:
- 9 April 2024 : Starting a trial/logistics
- 4 June 2024 : Running a trial - Part 1: Protocol compliance, monitoring
- 3 September 2024: Running a trial - Part 2: Recruitment and follow up
- 5 November 2024: Running a trial - Part 3: Data and Safety Monitoring Board (DSMB) options
28 January 2025: Closing down a trial and dissemination: Publication, including PPI in dissemination, media.
The final results of TB-PRACTECAL have been published in The Lancet Respiratory Medicine:
TB PRACTECAL is a phase 2/3 multicentre, randomised, controlled clinical trial to find short, tolerable and effective treatments for people with drug-resistant tuberculosis (DR-TB). The trial evaluated the efficacy and safety of three 24-week, all-oral regimens for the treatment of rifampin-resistant TB. These results confirm the interim results published in the New England Journal of Medicine in 2022:
A paper published in the Journal of Thrombosis and Haemostasis, by researchers from Semmelweis University in Hungary and the CTU reveals that anaemia can hinder blood clotting and potentially make tranexamic acid (TXA), a drug used to prevent postpartum haemorrhage (PPH) in women, less effective.
This study was conducted to determine the separate and combined role of TXA and red blood cells in the inhibition of fibrinolysis. Read the publication:
The WOMAN-2 trial is assessing whether giving TXA can prevent PPH in women with moderate and severe anaemia. The results of this trial are expected in 2024.
Important new results and health economic analysis from REVIVED-BCIS2 have been published.
A secondary analysis was published in JAMA cardiology:
These results were presented at the American College of Cardiology conference (ACC 2023) and revealed that percutaneous coronary intervention (PCI) remained ineffective, regardless of the extent of viability. In addition, the analysis showed that measuring scar burden is major predictor of events and left ventricular recovery.
A health economic analysis of data from REVIVED-BCIS2 was published in AHA Journals:
These results were presented at the American Heart Association conference (AHA 2023). This analysis showed that PCI was associated with minimal difference in QALYs and considerable incremental cost and that a strategy of PCI for ischemic left ventricular systolic dysfunction (ILVD) is not a justifiable use of healthcare resource.
The qualitative results of the Safetxt trial have been published in BMJ open: A qualitative study exploring experiences of the safetxt digital health intervention to reduce sexually transmitted infections in young people in the UK
Safetxt was a single blind randomised controlled trial and a linked qualitative study to establish the effectiveness of a safer sex intervention delivered by mobile phone messaging on STI infection.
For more details listen to the STIRIG series event.
The LSHTM Trials Network is holding an inaugural Trials Day as a hybrid event at Mary Ward House on Monday 6 November 2023. The day will showcase a selection of LSHTM’s vast experience and innovation in randomised controlled trials and there's a great line-up of speakers including keynotes by CTU members Eni Balogun on tranexamic acid trials and Richard Evans on cardiovascular trials
See the full agenda.
The WOMAN-2 trial has achieved its target of recruiting 15,000 women randomised across sites in Nigeria, Pakistan, Tanzania and Zambia.
The trial's aim is to see if giving tranexamic acid can prevent postpartum haemorrhage (PPH) and other severe outcomes in women with moderate and severe anaemia. Find out more about the trial.
The ARREST trial, involving the London Ambulance Service and all hospitals in London, found there’s no difference in 30-day survival for out-of-hospital cardiac arrest (OHCA) patients who were taken by ambulance to a specialist cardiac arrest centre compared with those delivered to the geographically closest emergency department. The study also found no overall difference in brain and nerve function at discharge and no difference between the two groups in death rates after three months.
The results were presented at the European Society of Cardiology Congress and published in The Lancet:
- Expedited transfer to a cardiac arrest centre for non-ST-elevation out-of-hospital cardiac arrest (ARREST): a UK prospective, multicentre, parallel, randomised clinical trial
- Supplementary appendix (pdf)
The trial’s aim was to determine the best post-resuscitation care pathway for patients without ST-segment elevation (STE). The ARREST trial involved 862 patients recruited across London between 2018 and 2022.
Read about the pilot:
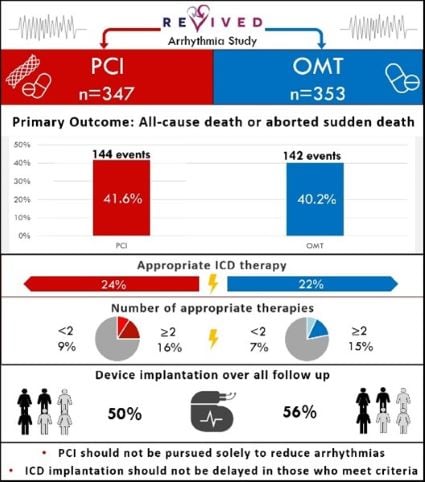
Further analysis from the REVIVED-BCIS2 trial shows:
- Percutaneous coronary intervention (PCI) does not reduce death or aborted sudden death in ischemic left ventricular dysfunction
- There’s no difference in the rate of implantable cardioverter defibrillator (ICD) therapies or sustained ventricular arrhythmias
Read the recent publication: Arrhythmia and Death Following Percutaneous Revascularization in Ischemic Left Ventricular Dysfunction: Prespecified Analyses From the REVIVED-BCIS2 Trial
REVIVED-BCIS2 evaluated the efficacy and safety of PCI compared to optimal medical therapy (OMT) alone for ischaemic left ventricular dysfunction. Seven hundred patients were enrolled across 40 centres in the UK between August 2013 and March 2020.
See the main trial results in the New England Journal of Medicine: Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction
Evidence from the WOMAN-2 trial shows that pregnant women with anaemia are substantially more likely to suffer life-threatening bleeding after childbirth. These findings are published in the The Lancet Global Health:
For further information listen to this podcast of Prof Rizwana Chaudhri and Prof Ian Roberts discussing their research investigating the association between maternal anaemia and post-partum haemorrhage from The Lancet Global Health.
Watch this video on: How maternal anaemia causes severe bleeding in childbirth
The WOMAN-2 trial recruited 10, 620 women with anaemia from hospitals in Nigeria, Pakistan, Tanzania and Zambia between August 2019 and November 2022. WOMAN-2 examined the association between anaemia and the risk of postpartum haemorrhage (PPH). The trial looks at whether giving tranexamic acid can prevent PPH and other severe outcomes in women with moderate and severe anaemia.
Read more about the WOMAN-2 trial here.