Search Centre projects
TB or not TB: What is Tuberculosis? Challenging the Current Paradigm of Tuberculosis Natural History using Mathematical Modelling Techniques
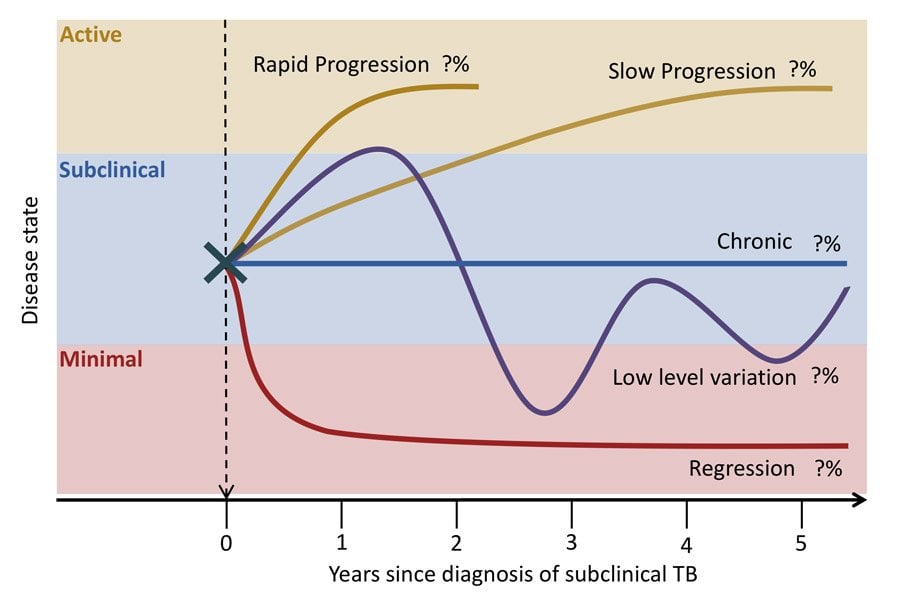
What is Tuberculosis (TB)? How does a person go from infection to disease, and what does this mean at the population level? Current models of disease typically account for two distinct stages of infection and disease, with mostly one-directional progression between them. Instead, data has shown that individuals experience a range of stages of disease intensity. Moreover, over time individuals can move between stages through a dynamic interplay between immune induced repression and disease progression.
This project will collate the best available data to parameterize a new mathematical model of TB with to capture the required dynamics. Using this structure, the project will explore critical questions in two areas of TB research the challenge of addressing latent tuberculosis infection; and incorporating the impact of changes in socio-economic indicators on TB trends.
Project dates: March 2018 - February 2023
Project lead and staff: Rein Houben, Jon Emery
Collaborating institutions: Vietnam NTP
Funding: European Research Council Starting Grant
TB PRACTECAL economic evaluation sub-study
The overall aim of this sub study is to estimate the probability that new MDR-TB regimens containing bedaquiline and pretomanid will be cost-effective from a societal as compared to the standard of care for MDR-TB patients in three settings: Uzbekistan, South Africa, and Belarus. A secondary aim is to assess the costs from a provider perspective of treating patients with these new regimens (new MDR-TB regimens containing bedaquiline and pretomanid), and estimate the impact of new regimens on prevalence of catastrophic costs due to TB.
Project dates: May 2018 - December 2021
Project lead: Sedona Sweeney
Collaborating institution and funder: MSF
The Role of Complement Component C1q in Tuberculosis and Diabetes Co-morbidity
Type 2 diabetes mellitus (T2DM) increases the risk of TB three-fold following infection, and although this epidemiological link has been recognised for over a century, the underlying causal mechanism linking the two diseases is still unclear. Further, TB patients with T2DM co-morbidity have worse treatment outcomes, including higher relapse and mortality rates than uncomplicated TB patients5.
The T2DM epidemic is rapidly growing and it is estimated that by 2040 there will be 642 million people living with T2DM worldwide6: it is also estimated that approximately one-third of the world’s population is infected with M. tuberculosis and already more TB cases are attributable to T2DM than to HIV. The development of interventions to prevent TB disease in T2DM patients are critical, but this is dependent on better understanding of underlying disease pathogenesis5. In this project, we propose to determine the role of C1q in TB/T2DM co-morbidity, as it is involved in immuno-pathology and inflammation in the two separate diseases and could be a causal link.
Project dates: September 2017 - February 2020
Project lead and staff: Jackie Cliff, JiSook Lee and Hazel Dockrell
Collaborating institutions: Universitas Padjadjaran
TIME country application
Application of the TIME modelling suite to inform TB policy decisions in high burden countries.
Project dates: December 2019 - March 2020
Project lead and staff: Rein Houben, Nabila Shaikh, Jamie Rudman
Collaborating institution: Avenir Health
Funder: GFATM
TRANSVAC2: European Network of Vaccine Research and Development
TRANSVAC2 supports innovation for both prophylactic and therapeutic vaccine development. High-quality technical services across four different service platforms are offered: Technology, Immunocorrelates & Systems Biology, Animal models, and support for Clinical Trials. Academic and non-academic research groups, including SMEs, can apply to benefit from the expertise, reagents, and facilities offered by TRANSVAC2 to accelerate the development of their vaccines. TRANSVAC2 also organises training activities on vaccine development-related topics.
Project dates: May 2017 - April 2022
Project lead and staff: Hazel Dockrell, Steven Smith
Collaborating institution: Led by EVI
Funder: EC Horizon2020 Infrastructures
TriageTB: Field evaluation of a point-of-care triage test for active tuberculosis
A point-of-care (POC), rapid, laboratory-free triage test for TB can potentially transform TB diagnostic services in resource-limited settings. A 4-marker host serum biosignature in AE-TBC and ScreenTB, previous EDCTP-funded projects. The multiplex test or a multi-biomarker test (MBT), consists of a single lateral flow (LF) strip, which measures individual concentrations of multiple biomarkers using upconverting reporter particles (UCP), within 30 minutes.
The performance of the biosignature will be validated on samples from Asia, South America and Eastern Europe for universal applicability and on samples from 900 newly recruited participants from Southern, West and East Africa in peripheral health care settings in a laboratory-free manner.
Project dates: March 2020 - February 2024
Project lead and staff: Hazel Dockrell (CoPI), Jayne Sutherland (CoPI)
Collaborating institutions: Stellenbosch University, Cape Town, South Africa; MRC Gambia, The Gambia, Makerere University, Uganda; LUMC; Leiden. The Netherlands; FIND, Geneva; Linq Management, Germany
Funder: The European & Developing Countries Clinical Trials Partnership (EDCTP)
Treatment outcomes and health inequalities in MDR-TB in Europe
Costs of MDR-TB drugs are higher in Eastern Europe compared to standard of living/GDPs. The new outcome measures which take into account treatment failure and relapse will compare data on access and relative cost of the new TB drugs.
Project dates: October 2017 - October 2021
Project lead: Graham Bothamley
Collaborating institutions: TBnet-ERS Clinical Research Collaboration: AIGHD, Amsterdam, Netherlands; RRPCPTB, Belarus; DGHSM, Harvard, USA; Research Centre, Borstel, Germany; San Rafaele, Milan, Italy; KKJM, Bochum, Germany; ICDDRB, Dhaka, Bangladesh; LIIU, Cape Town, South Africa; REUH, Riga, Latvia; NRCM/MSF, Paris, France; PDCH, Porto, Portugal
Funder: European Respiratory Society (ERS)
Tuberculosis Reduction through Expanded Antiretroviral Treatment and Screening for active TB (TREATS)
TREATS has been set up by a consortium of organisations that is already running the largest ever trial of a combination HIV prevention strategy. This trial, called HPTN071, or PopART, is being conducted across 21 communities in Zambia and South Africa, covering around one million people in total. PopART involves universal testing and treatment for HIV through house-to-house visits across the 21 communities on an annual basis over four years -- from 2014 to 2018. As part of PopART, all members of these communities are also screened for TB.
Building on PopART, TREATS will measure the impact of a combined TB/HIV intervention – of population level active case-finding for TB, combined with universal testing and treatment for HIV – on TB incidence, prevalence and incidence of infection.
Project dates: November 2017 - October 2021
Project lead and staff: Helen Ayles (L), Richard Hayes, Sian Floyd, Virginia Bond, Albertus Schaap, Barry Kosloff, Maria Ruperez, Lily Telisinghe
Collaborating institutions: Health Systems Trust (South Africa), Zambart (Zambia), KNCV Tuberculosis Foundation, University of Sheffield, LSE, Imperial College, The Union, Qiagena and Delft Imaging Systems
Funder: EDCTP
Factors influencing BCG-mediated in vitro mycobacterial growth inhibition in infants from Uganda
Tuberculosis (TB) is caused by Mycobacterium tuberculosis (M.tb), a facultative intracellular pathogen. This disease is the leading cause of death from a single infectious microbe in the world. The vaccine, Bacillus Calmette–Guérin (BCG) is used to protect against TB and is given to infants at birth. Although it is able to prevent disseminated forms of TB in childhood, BCG mediated protection against pulmonary TB, the most common form of the disease, is reduced in equatorial regions.
The reasons for the suboptimal performance of this vaccine in the tropics are unknown, however, it is hypothesised that exposure to mycobacteria in the environment, infection with parasites and viruses may negatively impact on BCG immunogenicity. Furthermore, it is proposed that transplacentally transferred maternal antibodies may impair the development of immune responses to BCG in infants when given at birth. Vaccine-mediated mycobacterial growth inhibition is a functional measure of the immune responses and has the potential to predict vaccine efficacy. We propose to investigate the impact of early infections and maternal antibodies in infants on in vitro mycobacterial growth inhibition. This may help us identify some of the causes of the low protection provided by BCG in the tropics.
Project dates: June 2019 - February 2020
Project lead: Simon Kimuda (L), Rein Houben, Richard White, Liz Corbett
Collaborating institutions: Vietnam NTP
Understanding and modelling sex disparities in TB
Men’s disadvantage in TB is clear: 70% of undiagnosed cases, 60% of notified cases, 65% of deaths. Gaps in detection and reporting are greater for men than women, and men are less likely than women to complete treatment. Our modelling work explores how strategies to reduce the gender gap in TB care by improving men’s access to diagnostic and treatment services could impact TB incidence and mortality in men, women, and children.
Project lead and staff: Katherine Horton (L), Rein Houben, Richard White, Liz Corbett
Collaborating institutions: Vietnam NTP
Funding: Bill & Melinda Gates Foundation