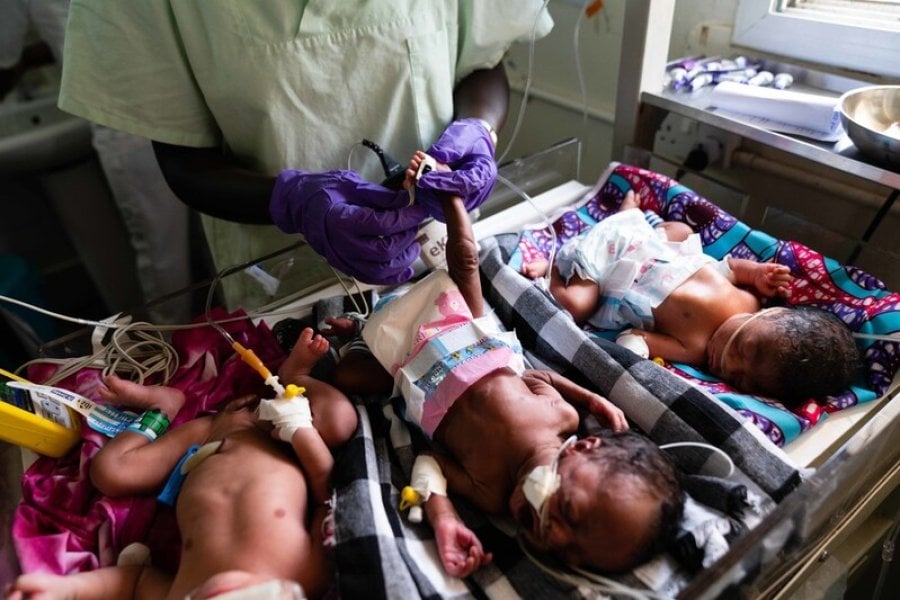
The pager goes off, it’s the delivery ward. You head over to find out where you are needed, it is a 25-year-old pregnant woman in labour with a baby that is coming 10 weeks too early. The woman has had a fever for the last 2 days, confusion and collapsed today. Her family brought her to Kampala’s main hospital and here she went into labour. They tell you that she was vomiting with abdominal pain for the last few hours. As a paediatrician attending this delivery, you are now worried that that baby may have the same infection as their mother, and added to that complication they are going to be born prematurely. As soon as they are born, you stabilise them and take them to the intensive care baby unit and start IV fluids and antibiotics. You follow the WHO AWaRe guidelines for neonatal sepsis, intravenous amoxicillin and gentamicin, but know that these antibiotics rarely work in these babies. You wish you had something better to offer.
Prevalence of antimicrobial resistance (AMR) varies geographically, and practitioners working in areas with high AMR rates are increasingly having to deal with the impact of this. Neonatal sepsis, affecting newborns and babies up to one month old, remains a significant cause of death in the first month of life in low resource settings (LRS). This is due to a number of factors, but increasing AMR in these settings means that WHO guidance on antibiotic choices is fast becoming ineffective, with few alternative options under development or in clinical trials. The NeoOBS study, a global, prospective, multi-centre study across 19 hospitals in 11 countries (predominantly Africa and Asia), has set out to establish the extent of this issue.
The study assessed antibiotic management strategies and outcomes of 3,204 hospitalised neonates and young infants (<60 days) with sepsis between 2018-2020. The study identified that empiric antibiotic use was hugely variable across the settings, with 206 combinations of antibiotics used and frequent escalation to alternative regimens. Only 39.7% of babies were started on WHO-recommended regimens, 25.9% first line (amoxicillin/gentamicin Group 1 Access) and 13.8% second line (cefotaxime/ceftriaxone, Group 2 “Low” Watch) regimens. Another 34.0% were started on regimens with extended-spectrum beta-lactamase (ESBL)/pseudomonal cover (piperacillin-tazobactam, ceftazidime, or fluoroquinolone based, Group 3 “Medium” Watch). Eighteen percent of babies had treatment initiated with carbapenems (Group 4 “High” Watch) and colistin-based regimens were started in 1.8% (Group 5 Reserve). This divergence from international standard of care guidance outlined by the WHO is worrying, but in many ways understandable, given the concern about local AMR patterns.
Appreciating the incidence of AMR pathogens causing sepsis in these regions is key to developing new locally-tailored empiric antibiotic trials, and ultimately WHO guidance, with the extent and type of AMR varying between countries. Overall, one fifth of the babies had positive blood cultures, with 2/3 of these being Gram-negative bacteria (commonest Klebsiella pneumoniae n=132, Acinetobacter spp. n=72 and Escherichia coli n=47) and 1/3 Gram-positive bacteria (commonest Coagulase negative staphylococci, CoNS n=84, Staphylococcus aureus n=54). It is easy to see why clinicians opt for non-ampicillin based regimens with these top five pathogens all resistant and varying efficacy of aminoglycosides including amikacin. Alarmingly, carbapenem resistance was >70% in Acinetobacter spp. and >30% in K. pneumoniae.
Clearly, new empiric antibiotic choices are needed for these vulnerable infants, whose mortality was 17.7% in pathogen-positive cases, with worse outcomes in Gram-negative sepsis. This study has helped to understand the epidemiology of neonatal sepsis in LRS and current practice, which should be used to identify potential safe and effective antimicrobial agents to use in these babies. Now we need to consider the repurposing of old drugs, and development of new and combination therapies to trial in babies in LRS to improve outcomes for those infected in the future.
Our postgraduate taught courses provide health practitioners, clinicians, policy-makers, scientists and recent graduates with a world-class qualification in public and global health.
If you are coming to LSHTM to study a distance learning programme (PG Cert, PG Dip, MSc or individual modules) starting in 2024, you may be eligible for a 5% discount on your tuition fees.
These fee reduction schemes are available for a limited time only.
