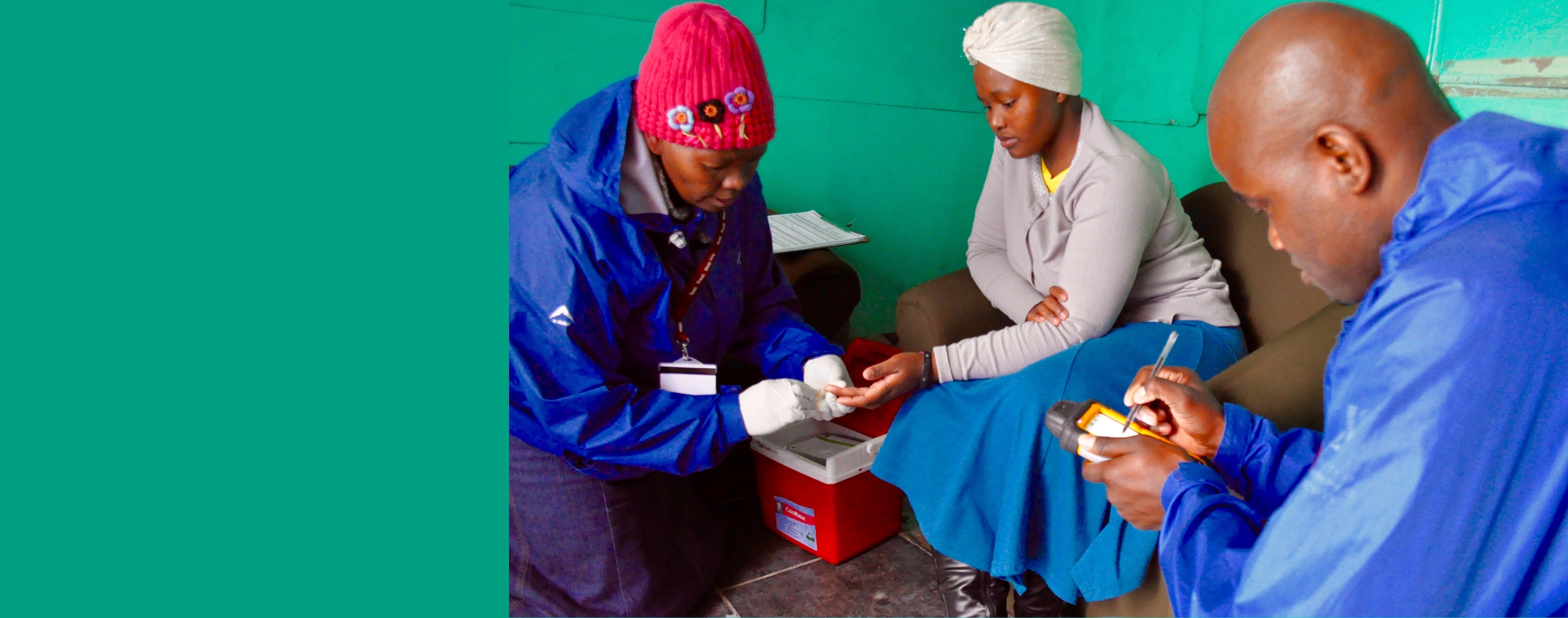
PopART is the largest community-randomised trial evaluating the impact of a combination prevention package including universal testing and treatment in reducing new HIV infections.
The study is being carried out by the London School of Hygiene & Tropical Medicine in collaboration with the HIV Prevention Trials Network (HPTN) and other partner organisations.
The HPTN 071 study, known as PopART, is the largest community-randomised trial of the universal HIV test and treat strategy. It is being conducted across 21 high HIV-burden, resource-limited urban settings in Zambia and Western Cape, South Africa, with a total population of about 1 million.
HIV incidence rates remain at very high levels in many parts of southern Africa and there is an urgent need for more effective HIV prevention strategies. Findings from HPTN 071 (PopART) will help inform scale up of future HIV programmes and help identify cost effective interventions.
This study will be critical for policy makers in determining whether the package of prevention interventions used in PopART will work at population level and whether it is cost-effective. In particular, it will find out whether a universal test and treat strategy – ensuring that everyone in the community knows their HIV status and offering immediate treatment for all those with HIV – is effective in reducing new HIV infections.
The study is being carried out in 21 communities (12 communities in Zambia and 9 in the Western Cape province of South Africa) from 2013 to 2018. Part of the HIV Prevention Trials Network, PopART is led by investigators from the London School of Hygiene & Tropical Medicine in collaboration with Imperial College London, Oxford University, Zambart, Lusaka, Zambia and the Desmond Tutu TB Centre at Stellenbosch University, South Africa.
The study is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) with funding from the U.S. President's Emergency Plan for AIDS Relief (PEPFAR). Additional funding is provided by the International Initiative for Impact Evaluation (3ie) with support from the Bill & Melinda Gates Foundation, as well as by NIAID, the National Institute on Drug Abuse (NIDA) and the National Institute of Mental Health (NIMH), all part of NIH.
Study design
A total of 21 communities (12 in Zambia and 9 in South Africa) were selected for study. The cluster or community for the purposes of this trial is defined as the catchment population of a local health unit, through which antiretroviral treatment (ART) for HIV is delivered. These 21 communities have been grouped into 7 matched triplets (4 in Zambia and 3 in South Africa).
Study communities were randomly assigned to one of three study arms. In the original design of the study, Arm A received the full PopART HIV combination prevention package, Arm B received the PopART package but with HIV treatment only offered to those eligible according to national guidelines, and Arm C received the current standard of care. In late 2015, in response to mounting evidence of clinical benefit, the World Health Organization (WHO) guidelines on HIV/AIDS were revised to recommend ART for all people living with HIV. The PopART study team responded by incorporating this recommendation into the study design, making ART available for all people living with HIV in all study arms. In Arms A & B, Community HIV-care Providers (CHiPs) will deliver the full PopART intervention package (see below).
To measure the impact of the intervention, a Population Cohort, consisting of a representative sample of approximately 2,000 adults aged 18–44 years, has been recruited from the general population of each of the 21 communities (an overall total of around 42,000 across all communities) and will be followed up once a year for three years to measure HIV incidence and other outcomes.
PopART will allow researchers to see if universal voluntary HIV counselling and testing, combined with the offer of immediate ART for those who test HIV-positive, has the potential to significantly reduce HIV incidence at the population level. In addition, research is being carried out to examine the acceptability of the PopART intervention and to document the effects of the interventions on a number of factors, including risk behaviours, social networks, HIV identity and community-level HIV associated stigma. Economic evaluations will measure the incremental cost of the intervention packages and will assess the burden on local health centres of implementing them.
For more detailed information about the study design please take a look at our publications or visit https://hptn.org/research/studies/137
The PopART intervention
The PopART intervention is a combination prevention package and includes the following HIV prevention methods:
- Offering universal voluntary HIV counselling and testing, delivered in the home by community health workers
- Referring people who are HIV positive to HIV care at their local health centre
- Offering immediate antiretroviral therapy (ART) irrespective of CD4-count, with potential benefits for the individual who has HIV and to prevent their partner(s) acquiring HIV
- Referring men who test HIV negative to professional medical male circumcision providers
- Promoting treatment for pregnant women with HIV, to prevent them from passing HIV to their babies
- Referring[dw1] individuals with symptoms suggestive of TB or sexually transmitted infections for diagnosis and care at a local health facility
- Providing condoms in the community
- The door-to-door PopART services are delivered by trained lay health workers called Community HIV-care Providers (CHiPs), while treatment and clinical care are provided by existing health systems in-country, with support from PopART.
Background
Recent clinical trials have demonstrated that measures included in the combination prevention package can be effective. While most of these prevention approaches are now offered in many settings with high rates of HIV, achieving high enough coverage to reduce the number of new infections has proven challenging. The PopART study aims to deliver the prevention package to all households within a community to enhance coverage and thereby reduce new HIV infections at the population level. Specially-trained community health workers will play a key role in achieving high coverage for these measures.
The PopART study builds upon previous research that supports the argument for this prevention approach. Previous trials (HPTN 052 and HPTN 043) have shown that treating an HIV-infected individual with ART can significantly reduce the risk of sexual transmission of the virus to an uninfected partner, and that providing community mobilisation and mobile HIV counselling and testing can improve rates of testing in rural communities, compared to settings where testing only takes place at clinics.
LSHTM research team
Richard Hayes, Professor of Epidemiology and International Health at LSHTM, is the Principal Investigator (PI) of the HPTN 071 (PopART) trial.
Helen Ayles is a Professorat LSHTM, Director of Research at Zambart, and Zambia PI for the HPTN 071 (PopART) trial.
Sian Floyd is an Assistant Professor in Epidemiology and Medical Statistics at LSHTM and the Senior Statistician for the HPTN 071 (PopART) trial.
Kalpana Sabapathy is a Clinical Epidemiologist at LSHTM. She is LSHTM’s Trial Coordination Associate for the HPTN 071 (PopART) trial and is also leading a series of nested case-control studies.
Ab Schaap is a Statistics Research Fellow and Data Specialist at LSHTM. Based in Zambia, he heads the Data unit at Zambart and is the Zambia Data Manager for the HPTN 071 (PopART) trial.
Barry Kosloff is Laboratory Manager at Zambart . He is the Zambia Laboratory Coordinator on the HPTN 071 (PopART) trial.
James Hargreaves, Professor of Epidemiology and Evaluation and Director of the Centre for Evaluation at LSHTM, is a Social Epidemiologist and co-investigator on the HPTN 071 (PopART) trial. He is also the PI for the HPTN 071 (PopART) Stigma Ancillary Study.
Shari Krishnaratne is a Social Epidemiologist who works mainly on analyses related to stigma.
Virginia Bond is a Social Anthropologist based in Zambia. She is Head of the Social Science unit at Zambart, and one of the Zambian co-investigators on the HPTN 071 (PopART) trial.
Janet Seeley is Professor of Anthropology and Health at LSHTM and advises on social science aspects of the HPTN 071 (PopART) trial.
Lily Telisinghe is a Clinical Epidemiologist based in Zambia where she is helping to coordinate TB-related research nested within the HPTN 071 (PopART) trial.
Project management team
Lucy Bradshaw is an Overseas Projects Manager at LSHTM. She oversees the contractual and financial management of the HPTN 071 (PopART) trial.
Danielle Wagner is a Project Manager at LSHTM. She provides the day-to-day project and financial management of the HPTN 071 (PopART) trial.
Alexandra Miller is Project Manager at LSHTM. She provides administrative support to LSHTM staff based at Zambart, Zambia.
F1000 (Tony Harries and others) have recommended our research article in the New England Journal of Medicine (NEJM): Effect of Universal Testing and Treatment on HIV Incidence — HPTN 071 (PopART).
- Dr Richard Hayes and Dr Sarah Fidler presented HPTN 071 (PopART) Trial: Impact of a UTT Intervention on HIV Incidence
- Dr William Probert presented Model Projections of the Impact of the PopART Intervention
- Dr Katharina Hauck presented Cost and Societal Value of the Household-Based Combination HIV Prevention Intervention Implemented in HPTN 071 (PopART)
- Dr Musonda Simwinga presented Community and Stakeholder Engagement in the HPTN 071 (PopART) Trial
The Clinical Trials Distance Learning Programme at the London School of Hygiene and Tropical Medicine (LSHTM) is celebrating International Clinical Trials Day with a focus on the HPTN 071 (PopART) Trial, a landmark trial evaluating whether universal testing and treatment for HIV is an effective prevention measure.
Impact of universal testing and treatment in Zambia and South Africa: Results of a community-randomised trial. Presented at CROI: Seattle 6th March 2019
Results from largest ever HIV prevention trial (HPTN 071 – PopART) suggest strategy could make a significant contribution to controlling epidemic
New HIV infections in southern Africa could be reduced substantially by offering entire communities voluntary HIV testing, and immediately referring those who test positive for HIV treatment in line with local guidelines, according to new research presented at the Conference on Retroviruses and Opportunistic Infections (CROI) in Seattle, USA.
The PopART study team attended the AIDS2018 conference in Amsterdam in July 2018 and gave a number of very interesting presentations. Find out more in the summaries of these presentations.
Following the AIDS2018 debrief in August, three presentations providing updates on PopART and the combination prevention trials - Implications for policy and practice, are now available.
With Thanks to the organizers: UNAIDS Science now and WHO HIV Knowledge series 2018, in collaboration with the UNAIDS FTI VTeam.
The proportion of people aware of their HIV status and receiving treatment has increased significantly after one year of a major HIV prevention programme, according to early findings published in PLOS Medicine.
Meera Senthilingam reports on a groundbreaking medical trial in South Africa.
- NPR: 'They Thought This HIV Strategy Couldn't Work. But It Did' (June 2019)
- HPTN Responds to Results from ANRS 12249 TasP Study Presented at AIDS 2016, July 2016
- Immediate ART Available to All People Living With HIV in HPTN 071 (PopART) Zambia Communities, May 2016
- Major HIV Population-Wide Testing and Early Treatment Study Launched, Sept 2013
- Major Grant Awarded for HIV Prevention Study in Africa, Sept 2011
P-ART-Y (PopART for Youth)
The PopART for Youth (P-ART-Y) study aims to evaluate the acceptability and uptake of the PopART combination prevention package among young people aged 10-24 years. Community HIV-care Provider (CHiP) data and findings from qualitative research will be used to determine whether the PopART intervention achieves adequate coverage of HIV testing and ART among young
people. If necessary, specially-targeted interventions for young people will be developed, implemented and evaluated. The P-ART-Y Study is being carried out in all 21 study communities in Zambia and South Africa and led by Kwame Shanaube.
A Comparison of Different Community Models of ART Delivery amongst Stable HIV+ Patients in Two Urban Settings in Zambia
The purpose of the study is to measure the virological and clinical outcomes of HIV+ patients offered two alternative models of ART delivery (ART adherence clubs or ART delivery by CHiPs) compared with usual standard of care in an urban setting in Zambia. In addition, it will measure the effect of the combined models on health facility crowding and waiting times. Laboratory data on viral suppression, clinical data on disease progression and qualitative methods will be used to answer the study questions. The study is conducted in the two PopART intervention communities in Lusaka, Zambia and led by Mohammed Limbada.
Case-control studies of factors associated with uptake of intervention
These two sub-studies are nested within the main PopART trial. In the first case-control study, 313 cases who declined the offer of HIV testing by CHiPs were compared with 329 controls who accepted testing. Cases and controls were interviewed to examine factors related to uptake of HIV testing. This study was carried out among cases and controls selected randomly from PopART Arm A and B communities in Zambia and South Africa.
In the second case-control study, 333 cases who were referred for HIV care by CHiPs but failed to initiate ART within 6 months were compared with 372 controls who successfully initiated ART within 6 months. Cases and controls were interviewed to examine factors related to successful linkage to care and rapid ART initiation. This study was carried out among cases and controls selected randomly from PopART Arm A communities in Zambia and S Africa. The study was led by Kalpana Sabapathy.
Economic Evaluation of PopART
The overall objective of the economic evaluation is to determine the cost-effectiveness of the PopART combination prevention package, compared with enhanced standard of care. To this end, the incremental benefits and costs of the interventions will be estimated. The team will estimate costs relating to door-to-door testing, prevention and treatment of HIV. Costs of the intervention will be estimated per community and per 10,000 population, to inform policy makers on financial implications of wider scale implementation. Cost data combined with the results of the trial and modelling projections will be used to estimate cost-effectiveness of the PopART intervention over different time horizons. The study is led by Katharina Hauck.
HIV self testing pilot and rapid impact evaluation
This implementation science study aims to address the limitations of the PopART door-to door strategy in reaching those who are as yet untested (mainly men). HIV self testing is a relatively new strategy that has now been endorsed by WHO. In recent studies this intervention has been found to be useful in reaching more first time testers, men and young people.
The study will pilot the offer of HIV self testing in addition to regular HIV testing and counselling by CHiPs in 4 of the intervention communities in Zambia. The study is led by Helen Ayles.
Modelling
The aims of the mathematical modelling work are to 1. Provide targets for the intervention, and revise these targets in light of accruing information during the trial; 2. Help interpret accruing information on intervention coverage by translating this into predicted impact, both for the benefit of the study team and for the DSMB; 3. Provide validated long-term projections on the regional effectiveness and cost-effectiveness of a PopART-like intervention implemented over different time periods; 4. Advance simulation science, and validate the use of mathematical models in HIV policy making by testing and improving models’ predictive power. The study is led by Christophe Fraser.
Phylogenetics
The phylogenetics study aims to help understand the patterns and sources of ongoing HIV transmission in the three study arms at the end of the HPTN 071 trial. Secondary aims include studying drug resistance, studying pre-trial incidence and contributing to the PANGEA consortium study.
In order to answer these questions, data will be collected from infected individuals in the population cohort and from patients recruited in health care facilities in 9 study communities in Zambia (3 triplets). Viruses will be genetically sequenced and analysed.
The study is led by Christophe Fraser.
Social science
The overarching aim is to maximize the transferability of study findings to other settings in the region. There are three specific objectives:
- A formative evaluation of the HIV-relevant contexts in all 21 study communities completed before trial commencement to inform study implementation – Broad Brush Surveys (BBS).
A multi-method, longitudinal description of the process of study implementation to document how the PopART intervention interfaces with real-world situational demands.
- An in-depth description of the experiences of families living in the study communities, particularly those living with HIV and members of key population groups, using ethnographic and participatory methods.
The Study is led by Virginia Bond.
HPTN071 Stigma Study
The study is a mixed and multi-method implementation science study of HIV-related stigma in communities and health settings. It investigates three hypotheses:
- The PopART intervention may change levels of HIV-related stigma.
- HIV-related stigma may undermine the effectiveness of the PopART intervention.
- The PopART intervention may change the forms of HIV-related stigma.
The team is conducting a 3-round open cohort study of all health workers working in or associated with health facilities, and of all the CHiPS workers, in all 21 study communities. From this group data is collected on their working lives and their perceptions and experiences relevant to understanding HIV and key population stigma in health settings. Finally, stigma is a core theme within qualitative research nested within PopART communities and services. The Study is led by James Hargreaves.
PopART in the news
- NPR: They Thought This HIV Strategy Couldn't Work. But It Did
- Johns Hopkins Bloomberg School of Public Health "Test and Treat" Thrives in New Territory
- HPTN Responds to Results from ANRS 12249 TasP Study Presented at AIDS 2016, July 2016
- Immediate ART Available to All People Living With HIV in HPTN 071 (PopART) Zambia Communities, May 2016
PopART press releases
- Major HIV Population-Wide Testing and Early Treatment Study Launched, Sept 2013
- Major Grant Awarded for HIV Prevention Study in Africa, Sept 2011
PopART videos
PopART podcasts
- HIV Treatment as Prevention in Africa, Feb 2014
- PopART: All-Out War on AIDS in Africa, Dec 2011
- 30th Anniversary of HIV/AIDS: Hope and Optimism, June 2011