On International Youth Day, we spoke to Dr Nyasha Dzavakwa, Clinical Lead at the VITALITY Trial, Zimbabwe, which aims to investigate the effect of providing vitamin D3 and calcium carbonate to children living with HIV.

What is the impact of HIV on the health of young people?
Before the arrival of antiretroviral therapy (ART), HIV had a devastating negative impact on young people causing severe morbidity and mortality. With the rapid scale up of ART, the survival of children living with HIV has improved so that children can now reach adolescence and adulthood. Thus, the focus has shifted from increasing survival to managing HIV associated comorbidities such as growth failure – particularly stunting and pubertal delay which have a negative effect on skeletal development.
What is the aim of the VITALITY trial?
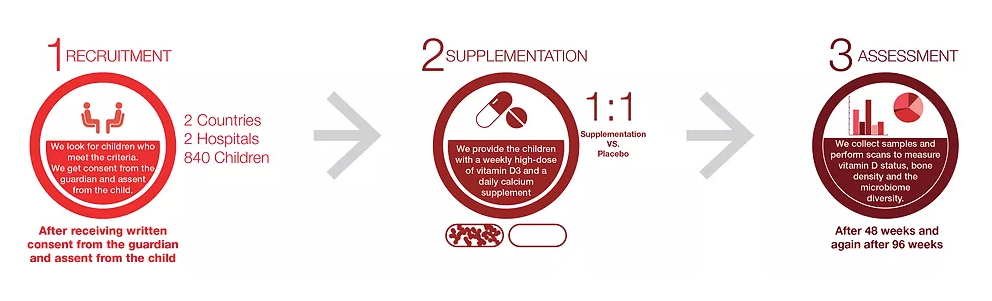
The VITALITY trial is a phase III clinical trial being conducted in Lusaka, Zambia and Harare, Zimbabwe. The main aim of the study is to determine the effect of weekly high dose vitamin D3 and daily calcium carbonate supplements on musculoskeletal health and immune regulation in 840 children with perinatally acquired HIV aged 11-19 years. The enrolled participants must have been established on antiretroviral therapy for at least 6 months. They are followed up for a total of 96 weeks, 48 weeks while receiving vitamin D3 and calcium carbonate intervention and an additional 48 weeks after the intervention to assess the sustainability of the intervention’s effects.
Why have you chosen Zambia and Zimbabwe?
Both Zimbabwe and Zambia are countries in sub-Saharan Africa, a region with the highest HIV epidemic in children and adolescents. This trial will have a higher impact in informing the need for public health interventions to monitor and treat musculoskeletal health problems in children living with HIV in this context. In addition, the chief investigator of the trial, Professor Rashida Ferrand, is based in Zimbabwe at The Health Research Unit Zimbabwe (THRU-ZIM) and we have enjoyed some previous fruitful research collaborations with Zambia.
Who are the trial partners?
We have tried to ensure that the VITALITY trial has wide north to south, north to north and south to south collaborations. Locally, in Zimbabwe, our trial partners include the Biomedical Research and Training Institute and The Health Research Unit Zimbabwe (THRU ZIM). In Zambia, the University Teaching Hospital and in Europe, University of Bristol, Research Center Borstel – Leibniz Lung Center, the University of Oxford and the London School of Hygiene & Tropical Medicine.
What does your role as clinical lead entail?
My role as a clinical lead involves ensuring that all trial procedures adhere to Good Clinical Practice (GCP) guidelines and principles across the two sites. I also ensure that the study procedures across the two sites are mirroring one another through regular phone calls, zoom meetings and exchange visits between the Zimbabwe and Zambia teams. These communication channels provide a platform to discuss and resolve any trial implementation issues. In Zimbabwe I also provide management and care to study participants who may fall ill whilst enrolled in the trial. In addition, I am responsible for organising the monthly trial management group meetings where I am the deputy chair, and the quarterly clinical trial effectiveness group (CEG) meetings.
What are the findings?
At the moment we do not have any preliminary results, but we are working on the analysis of our baseline data and this will be presented in a paper published at the end of this year.
Has COVID-19 had an impact on the project?
As a result of the COVID-19 pandemic and site-specific government-imposed lockdown measures we had to delay the trial start dates across the two sites from 2020 to 2021. Our trial drugs were also delivered late from the pharmaceutical company in India as flights were grounded. However, both sites managed to achieve target participant recruitment in record time despite many months of hard lockdown.
What are the next steps?
We have completed participant recruitment and are now conducting follow-up visits to our study participants. About 50% of the study participants have completed week 48 visits across the two sites. We do not have any participants who have completed the full 96 weeks yet, but we project that we will complete this in September 2023. With regards to our data analysis keep an eye out for the VITALITY baseline paper before the end of this year.
How can people find out more about the project?
More information about our study can be found on our Twitter page: @TrialVitality, on our website or on the THRU-ZIM website.
Alongside your role as Clinical Lead, you are undertaking a PhD. What are you investigating?
My PhD is focused on looking at interventions to improve treatment adherence in young people living with HIV at risk of virological failure in Harare, Zimbabwe. I am going to be using an electronic pill box called a ‘Wisepill RT2000 dispenser’. This device records the time and date every time its opened and this information is sent to a secure online server. For my study I will randomly select 200 young people living with HIV at risk of HIV virological failure (when ART fails to supress or sustain a person’s viral load to a safe level). Half of these will receive the intervention and the other half will receive standard care. The 100 intervention group participants will be asked to use the Wisepill RT2000 device for six months which will be linked to their mobile phones so that they can receive text message reminders every time a ‘no pill box opening event’ is recorded on the server at their schedule time for taking their ART drugs. Adherence is measured by viral suppression and will be compared the between the two groups.
Our postgraduate taught courses provide health practitioners, clinicians, policy-makers, scientists and recent graduates with a world-class qualification in public and global health.
If you are coming to LSHTM to study a distance learning programme (PG Cert, PG Dip, MSc or individual modules) starting in 2024, you may be eligible for a 5% discount on your tuition fees.
These fee reduction schemes are available for a limited time only.