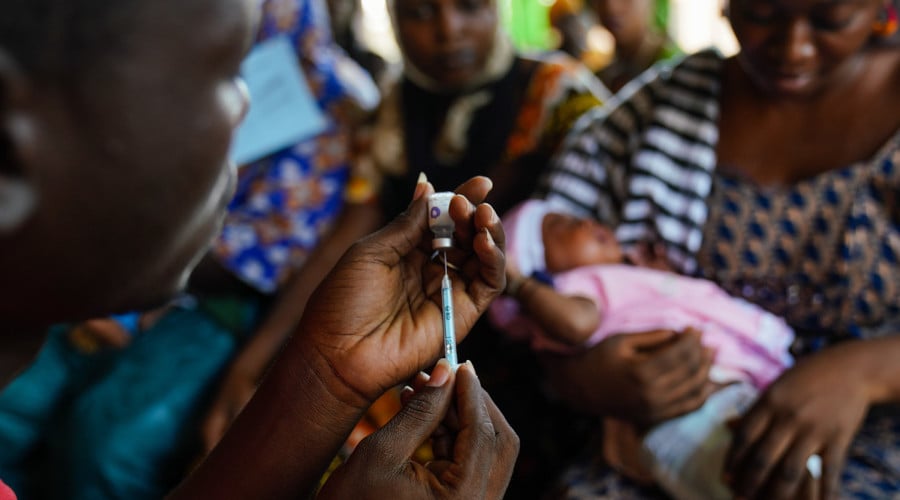
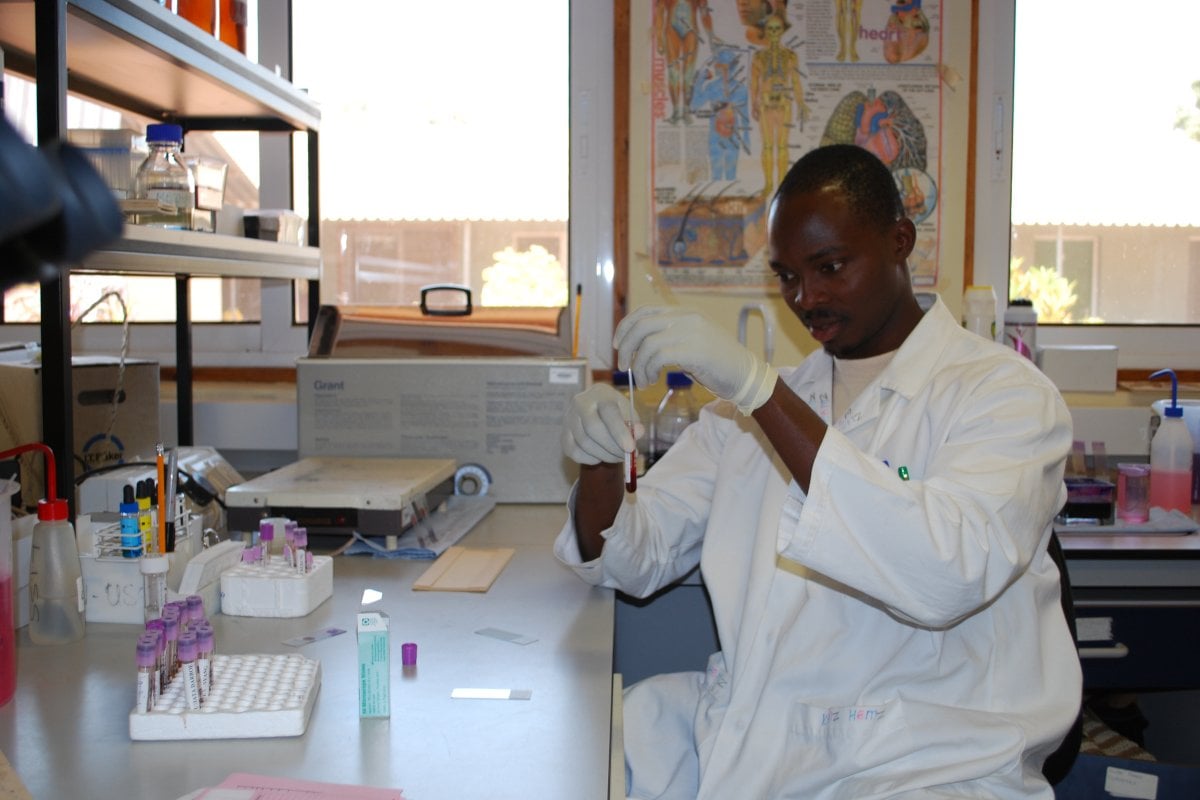
The vaccines and immunity theme works towards a better understanding of natural or vaccine-induced immunity implicated in defence against serious infectious diseases, including tuberculosis. Our studies in lab and field aim to inform the design of novel vaccines and maximise vaccine impact. We have developed a portfolio of discovery science and impact projects around this ambition. Using the core support structures and the diversity of skills of the leading investigators within the theme, we place the following questions at the centre of our work:
- What kind of immune responses should vaccines elicit to induce maximal protection?
- Can we identify correlates of protection urgently required to develop novel vaccines against pathogens requiring elimination by cell mediated immunity, e.g. M.tuberculosis
- Which vaccines are safe, immunogenic and effective in the long term in resource poor settings and how are they best used within the EPI program?
Through the conduct of both laboratory science and clinical trials, we aim to contribute to the evidence based development and delivery of vaccines. We aim to enhance the value of our clinical trials by gaining mechanistic insights into immune responses and age-dependent immune development in the context of vaccination, infection and important epidemiological and pathogen-derived co-factors. In the laboratory, we employ cutting edge immunological and systems biology tools to dissect compartments of the immune response in the context of infection and vaccination. In addition to the clinical trials, our field studies follow longitudinal observational cohorts. These include entire households and mother/infant pairs as platforms to investigate host responses in individuals of different ages, and to dissect the interactions between host and pathogen under vaccine or disease pressures.