The purpose of the Scientific Coordinating Committee (SCC) is to determine the scientific value, feasibility and methodology of proposed research projects and of on-going studies according to its terms of reference (TOR). It also assesses whether it aligns to MRCG at LSHTM's research priorities and objectives. The committee consists of scientists and heads of research related departments – Gambia Government representative is being invited to these meetings. The SCC is chaired by the Unit Director/ Disease control and elimination theme leader, Professor Umberto d'Alessandro.
Procedures for applicants for review by SCC and Ethics Committee
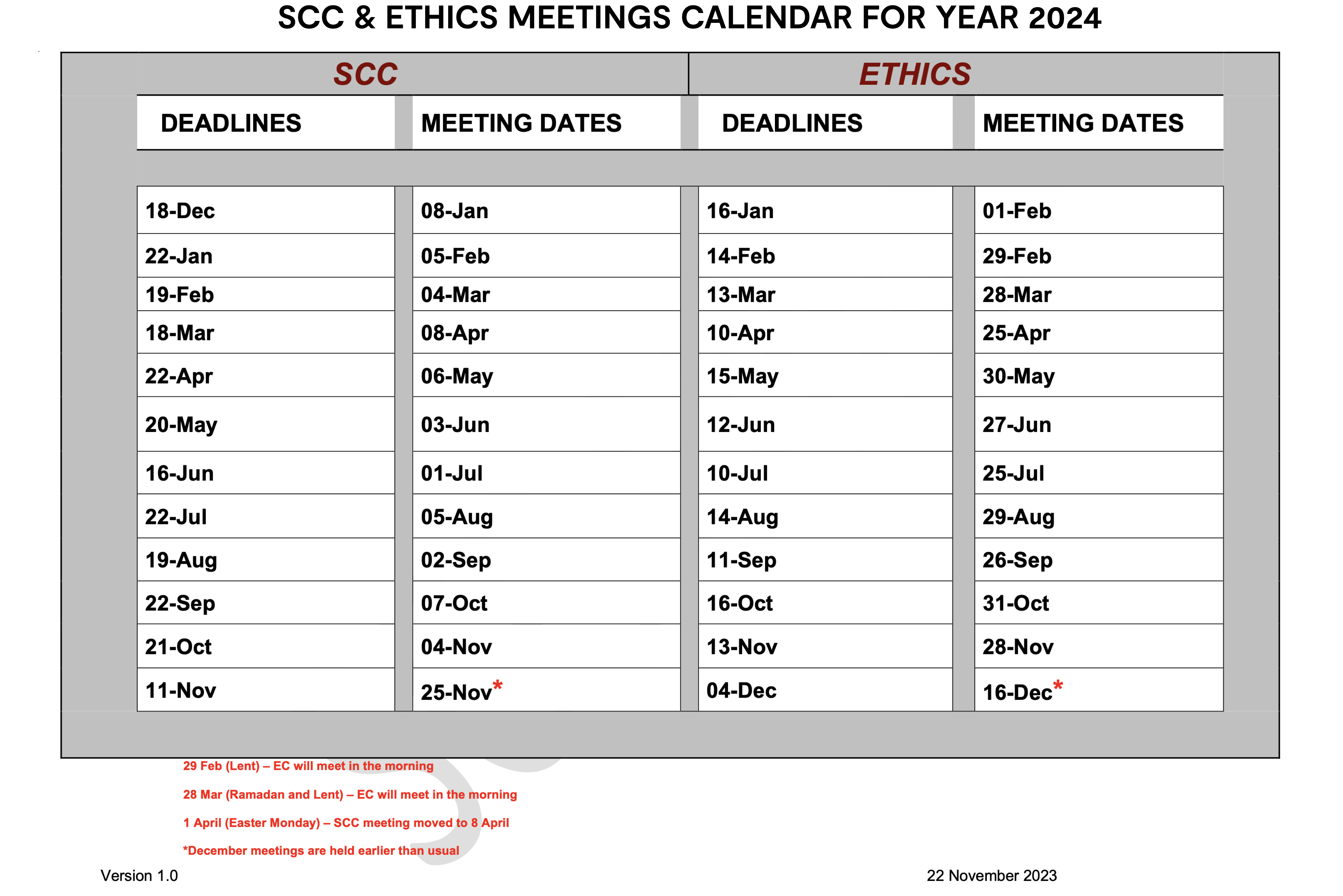
The deadline for submissions by applicants is 14 days before the actual meeting of the SCC. The dates of the deadlines and meetings are indicated in the Calendar below. Please note that applications received after the submission deadline will be held over to the following meeting.
New projects (proposals) including ancillary studies are to be submitted by completing the online form i.e. London Ethics Online (LEO) application:
Further guidance on how to complete the LEO form, how to submit other requests and notifications:
https://leo.lshtm.ac.uk/Personalisation/DisplayPage/9
The resources needed for a research activity is reviewed by the Research Support Office (RSO) and if the resources are not approved by RSO, the SCC Administrator will not validate the application for review by SCC members.
The meetings of the SCC are held on the first Monday of every month at 0830 hours at MRCG Fajara except January. The Principal Investigator (PI) or his/her representative will be invited to present the proposal at the SCC meeting.
Some days after the meetings, each applicant will receive a letter from the Chair communicating the decision of the committee. Submissions involving human participants or their samples or data that are approved by the SCC are being forwarded directly to the Ethics Committee (EC) for review.
The Ethics Committee sits on the last Thursday of every month at 1600 hours at MRCG, Fajara except January. The EC will review an application if it has been reviewed and approved by a recognised Science Committee in The Gambia, e.g. SCC of MRCG or Research & Publication Committee (RePubliC) of the University of The Gambia.
Some days after the EC meeting, each applicant will receive a letter from the Chair communicating the decision of the Ethics Committee.