Evaluating the impact of eave tubes plus house screening on malaria transmission
Location of study: Bouake, Cote d’Ivoire
LSHTM Investigators: Jackie Cook, Raphael N’Guessan, Immo Kleinschmidt, Welbeck Achille Oumbouke
External collaborators: Matt Thomas (Penn State, US), Eleanore Sternberg (Penn State, US), Serge Assi (Institute Pierre Richet, Cote d’Ivoire), Alphonsine Koffi (Institute Pierre Richet, Cote d’Ivoire), Marit Farenhorst (In2Care, The Netherlands), Anne Osinga (In2Care, The Netherlands), Malal Diop (In2Care, The Netherlands)
Funding Body: Bill and Melinda Gates Foundation
Vector control has resulted in large reductions in malaria transmission across sub-Saharan Africa; however, in some countries transmission remains high and insecticide resistance threatens current progress. Eave tubes are a novel vector control tool which aims to overcome resistance through bringing mosquitoes into contact with a high dose of insecticide as they try to enter a household. This project aims to evaluate the impact of eave tubes on epidemiological and entomological outcomes. A large cluster randomised control is currently taking place in Bouake, central Cote d’Ivoire, an area with high levels of insecticide resistance.
The trial incorporates 40 villages (20 per arm), with households in the intervention arm receiving screening and eave tubes, with an insert with a high dose of pyrethroid inserted, as well as receiving new long-lasting insecticide treated bed nets (LLIN). The control arm households have received LLIN. The trial involves an active case cohort in children aged 6 months to 10 years who are visited every two weeks during the transmission season, and every month during the dry season. During the visit, an RDT is taken if the child is febrile and a blood spot sample is taken for future analysis. The trial will run for two years. Secondary outcomes include malaria infection prevalence, measured in a cross-sectional survey 18 months post-installation of eave tubes; mean mosquito density and entomological inoculation rate (EIR). There are also important anthropological and economical considerations in this new type of vector control which need to be explored.
Evaluation of a novel long lasting insecticidal net (treated with the synergist PBO) and a indoor residual spray product, separately and together, against malaria transmitted by pyrethroid resistant mosquitoes: a cluster randomised controlled trial
Location of study: Muleba district, Kagera Region, Tanzania
LSHTM Investigators: Natacha Protopopoff, Mark Rowland, Jacques Derek Charlwood, Alexandra Wright, Catherine Pitt, David Bath, Immo Kleinschmidt
External collaborators: Franklin W Mosha, Alphaxard Manjurano & Jacklin Mosha, (Kilimanjaro Christian Medical University College, Tanzania)
Funding Body: Global Health trial DFID/MRC/NIHR/ Wellcome Trust
This cluster randomised controlled trial aimed to evaluate the efficacy of four vector control interventions for reducing malaria transmission and controlling malaria vectors resistant to pyrethroid insecticide. We allocated 48 clusters to four intervention arms using a factorial design: 1/ a standard long lasting insecticidal net (Olyset Net), 2/ a LLIN incorporating the synergist PBO (Olyset Plus), 3/ a standard LLIN and long lasting indoor residual spray formulation (Actellic 300CS), 4/ a PBO LLIN and Long-lasting IRS.
In the PBO LLIN arm the prevalence of malaria was reduced by 44% and 33% in the first and second year respectively compared to the standard LLIN arm treated with pyrethroid only. The addition of IRS to the standard LLINs provided a 44% additional protection against malaria compared to the standard LLIN alone, whilst the addition of IRS to PBO LLIN did not significantly improve protection over PBO LLIN alone. This was the first clear evidence that PBO LLINs are more effective than standard LLINs for malaria prevention in areas of high pyrethroid resistance and justifies their increased deployment and use. As a direct consequence of the trial, WHO gave interim endorsement to PBO LLIN in September 2017 as new WHO class of vector control product, and recommended that PBO LLINs are deployed for malaria prevention in areas where vectors are resistant to pyrethroids. Analysis of the third year trial data and laboratory testing of the residual bio-efficacy of the LLINs are ongoing.
Contribution of Plasmodium knowlesi to multi-species human malaria infections in North Sumatera, Indonesia
Location of study: North Sumatera Province, Indonesia
LSHTM Investigators: Inke Lubis, Paul Divis, Khalid Beshir, Colin Sutherland
External collaborators: Hendri Wijaya, Munar Lubis, Chairuddin P. Lubis (Department of Paediatrics, Faculty of Medicine, University of Sumatera Utara, Medan, Indonesia)
Funding Body: Directorate General of Higher Education, Indonesia
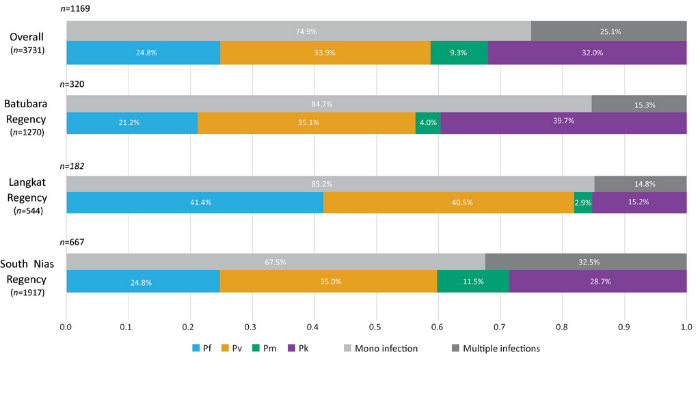
Advances in DNA-based detection of malaria parasites has enabled better understanding of the infections present in human communities, important information to assist malaria elimination. We tested volunteers in 3 Regencies in Sumatera and found a complex mix of four malaria species contributing to the burden of infection. These included the monkey parasite Plasmodium knowlesi. As Indonesia works towards the goal of malaria elimination, information is lacking on malaria epidemiology from some western provinces.
As a baseline for studies of antimalarial efficacy, we surveyed parasite carriage in three communities in North Sumatera Province. A combination of active and passive detection of infection was carried out among communities in Batubara, Langkat and South Nias regencies. Finger-prick blood samples from consenting individuals of all ages provided blood films for microscopic examination and blood spots on filter paper. Plasmodium species were identified by nested polymerase chain reaction (PCR) of rRNA genes, and a novel assay which amplifies a conserved sequence specific for the sicavar gene family of P. knowlesi. 614 of 3,731 participants (16.5%) were positive for malaria parasites by microscopy. PCR detected parasite DNA in samples from 1,169 individuals (31.3%). In total, 377 participants (11.8%) harboured P. knowlesi. Also present were P. vivax (14.3%), P. falciparum (10.5%) and P. malariae (3.4%). Amplification of sicavar is a specific and sensitive test for the presence of P. knowlesi DNA in humans. Subpatent and asymptomatic multi-species parasitaemia is relatively common in North Sumatera, and so PCR-based surveillance (PCR) -based surveillance is required to support control and elimination activities.
Program for Resistance, Immunology, Surveillance, and Modelling of malaria (PRISM2): Transmission Project
Location of study: Tororo district, Uganda
LSHTM Investigators: Chris Drakeley, Sarah Staedke
External collaborators: Teun Bousema and Chiara Andolina, (Radboud Institute for Health Sciences, Netherlands); Grant Dorsey, (University of California, San Francisco, USA); Moses Kamya, (Makerere University / Infectious Diseases Research Collaboration, Uganda)
Funding Body: UK Research Councils
A better understanding of how malaria is transmitted from human hosts to mosquito vectors is needed. We aim to characterize factors associated with gametocyte production, evaluate the impact of human and parasite factors on parasite infectivity to mosquito vectors, and characterize the human infectious reservoir for malaria in Tororo, Uganda. Between October 2017 and October 2018, we followed 492 participants from 80 randomly selected households in Tororo, Uganda. Participants are seen monthly to estimate the incidence of malaria and assess parasite densities in relation to characteristics of the human host and the infection.
Blood samples from individuals who were qPCR positive for P. falciparum at the prior monthly visit are fed to female An. gambiae mosquitoes, which are dissected 9-10 days later to examine for oocysts. Of 200 membrane feeding experiments, 26 (13.0%) resulted in mosquito infections. Stratified by clinical presentation, 13 of the 47 feeds (27.7%) done on asymptomatic participants with microscopic parasitaemia were infectious, compared to 11 of the 126 feeds (8.7%) done in individuals with sub-microscopic parasitaemia and 1 of 8 (12.5%) participants with clinical malaria. The quantification of male and female gametocytes and genetic characterization of gametocyte populations are ongoing. This unique study design, with intensive monitoring of natural malaria infections in an all-age cohort, will help us to understand the dynamics of gametocyte production and infectivity over time.
Predictive analysis across spatial scales links zoonotic malaria to deforestation
Location of study: Sabah, Malaysia
LSHTM Investigators: Kimberly Fornace, Jon Cox, Chris Drakeley
External collaborators: Paddy Brock, Heather Ferguson, Rowland Kao (University of Glasgow, UK); Timothy William (Infectious Diseases Society Kota Kinabalu, Malaysia); Matthew Grigg, Nicholas Anstey (Menzies School of Health Research, Australia)
Funding Body: UK Research Councils
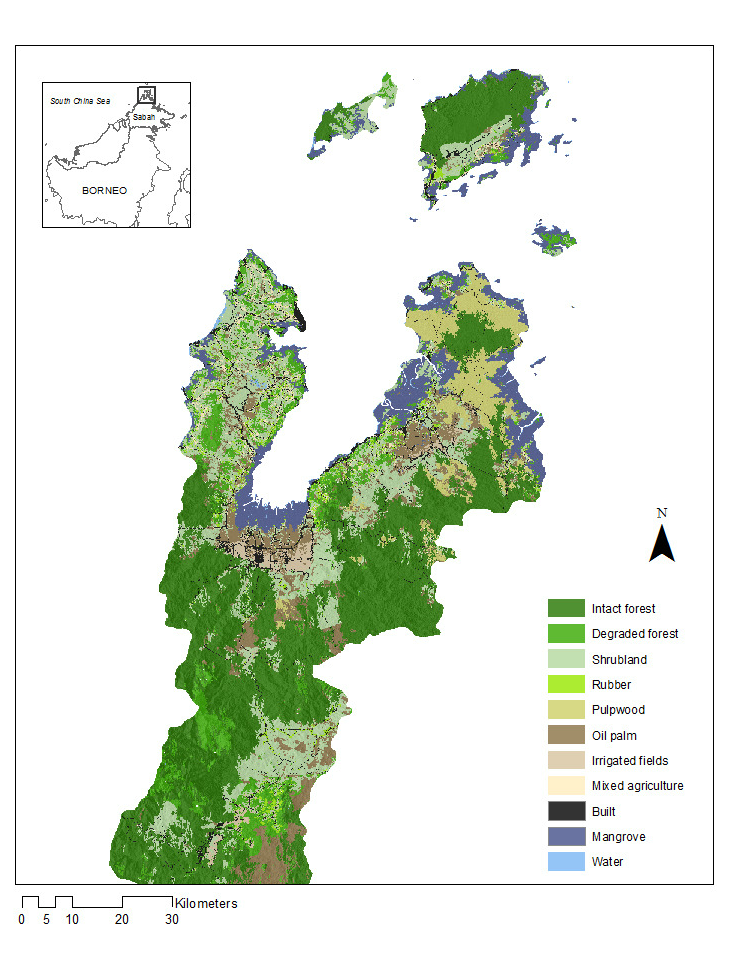
Incidence of the zoonotic malaria Plasmodium knowlesi has been linked to deforestation. As most people are infected outside of the house, household risk may be determined by environmental factors at different spatial scales due to the movements and distribution of people, mosquito vectors and wildlife hosts.
We utilise a machine learning pathway to identify landscape factors defining the relationship between zoonotic malaria and environmental change. Using data from satellite imagery, GPS locations of P. knowles cases and a cross-sectional survey, we fit models of household occurrence of P. knowlesi at 11 spatial scales, from 100m to 5km around the house. The proportion of cleared land around the household was most predictive at 1km while aspect, slope and canopy regrowth were all important at small spatial scales. In contrast, fragmentation of deforested areas influenced P. knowlesi at larger scales (4 and 5km). Models were most predictive at multiple spatial scales, illustrative of the complex nature of transmission of this disease and relationships with environmental change.
Rice and Malaria Vectors in West Africa
Location of study: Cote d’Ivoire
LSHTM Investigators: Jo Lines, Jeff Waage, Kallista Chan, Raphael N’Guessan
External collaborators: Kazuki Saito (AfricaRice, Cote d’Ivoire), Rousseau Djouaka (International Institute of Tropical Agriculture)
Funding Body: CGIAR Agriculture for Nutrition and Health Research Programme
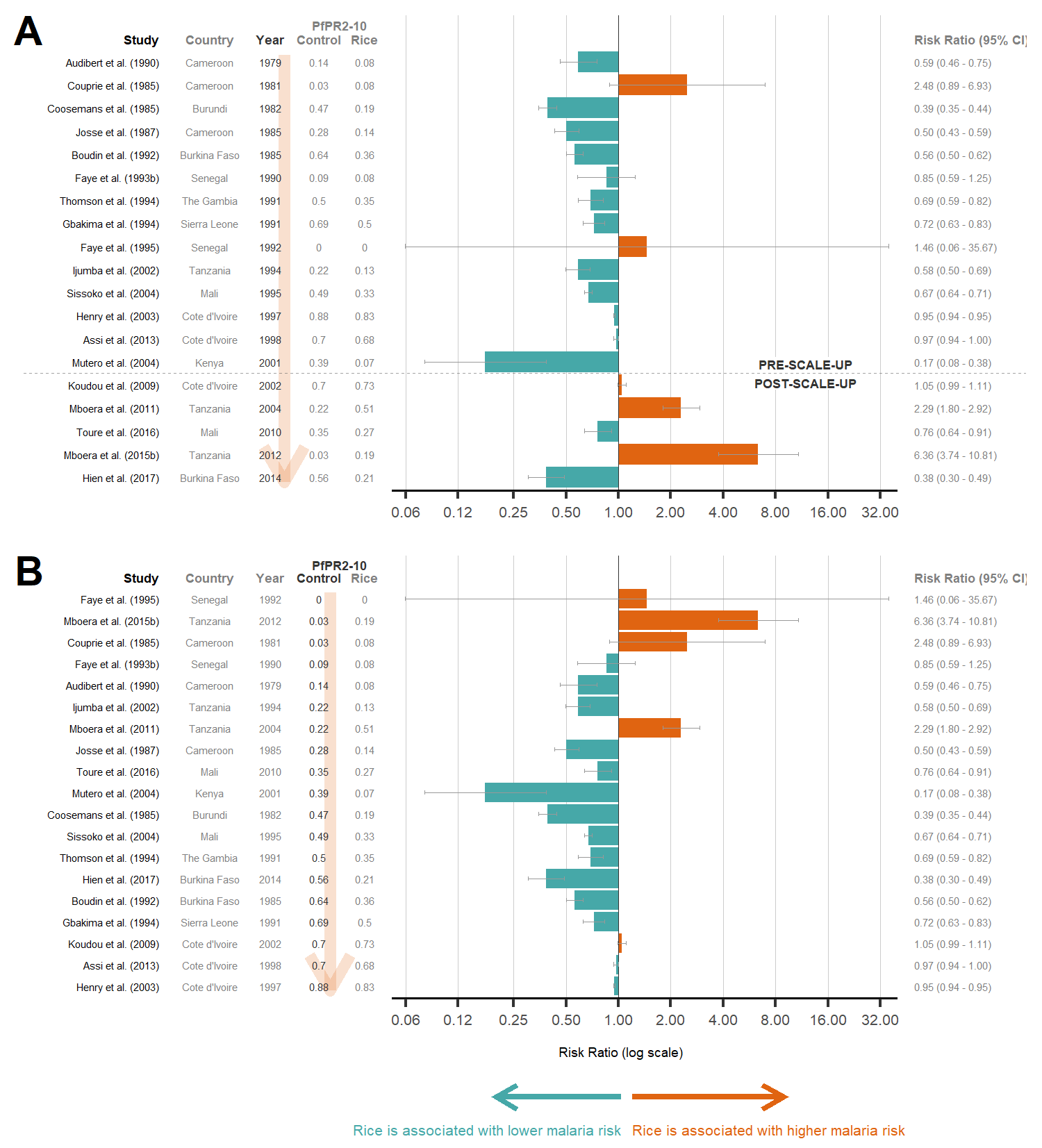
Rice fields are ideal breeding sites for African malaria vectors and can bring increased malaria risk amongst rice-growing communities. However, for a growing African population, rice cultivation is a necessity and cannot be stopped. Thus, to minimise malaria risk, we are searching for rice-growing techniques that can significantly minimise vector densities.
Rice can be grown in vast and variable methods and the techniques that could potentially affect vector densities are endless. Some of these techniques include water management (alternate wet-dry irrigation vs. continuous flooding), crop establishment, weeding, hoeing and pesticide and herbicide use. At present, the main outcomes of rice research include yield, water use and weed production. However, we want to find out how current and prospective rice-growing methods could affect (increase or decrease) malaria vector densities. Thus, we collaborate with AfricaRice on their on-farm trials, and include mosquito abundance as an additional parameter to record in their rice research. This will involve developing an efficient and representative method of sampling larvae in rice fields, and using that to estimate the adult vector productivity of rice fields. We will also explore rice farmers’ views and perspectives on rice cultivation and its effect on mosquitoes and health.
Validating serological markers of recent malaria exposure as measures of seroincidence
Location of study: The Gambia, Uganda
LSHTM Investigators: Lindsey Wu, Chris Drakeley, and Kevin K.A. Tetteh
External collaborators: Isabel Rodriquez, Bryan Greenhouse, Inna Gerlovina (UC San Francisco, USA) Isaac Ssewanyana (Infectious Disease Research Collaboration, Uganda)
Funding Body: UK Medical Research Council, US National Institutes of Health, Intellectual Ventures
Serology has been widely used to measure historical malaria exposure based on antibody responses to Plasmodium falciparum parasite proteins. Recent studies have also identified markers correlated with recent malaria infection via protein microarray. Using the Luminex quantitative suspension array technology (qSAT) and individual-level longitudinal data from The Gambia and Uganda, this project aims to evaluate a panel of novel Pf antigens and their accuracy as measures of sero-incidence.
With the Luminex multiplex immunoassay (MAGPIX) and a panel of over 20 Pf antigens, we quantified the temporal dynamics of antibody responses in an all-age longitudinal cohort of 2,688 individuals in rural Gambia. We estimated their sensitivity/ specificity for identifying rapid diagnostic test (RDT)- positive or polymerase chain reaction (PCR)-positive infections in the last 150 days. Combined antibody responses to several antigens showed high sensitivity/specificity as markers of Pf infection in the previous six months. Area under the curve (AUC) values ranged from 0.85 (95%CI 0.72-0.91) and 0.85 (0.77-0.92) to 0.77 (0.62- 0.82) in ages 1-5 years, 6-15 years, and >15 years respectively. Research is currently ongoing to compare these outcomes with longitudinal data in Uganda amongst individuals with confirmed malaria infection in the previous year. The predictive power of these serological markers is being assessed with respect to epidemiological covariates such as age, time since last infection, geographical region, parasite density, and repeat exposure.
Achieving Catalytic Expansion of Seasonal Malaria Chemoprevention in the Sahel (ACCESS-SMC)
Location of study: Burkina Faso, Guinea, Chad, Mali, Niger, Nigeria, The Gambia
LSHTM Investigators: Paul Milligan , Susana Scott, Matt Cairns, Colin Sutherland, Khalid Beshir, Julian Muwanguzi, Sham Lal, Paul Snell, Raoul Mansukhani, Yolanda Fernandez, Rhosyn Tuta
External collaborators: Malaria Consortium; Catholic Relief Services; Management Sciences for Health (MSH); Medicines for Malaria Venture (MMV); Institut de Recherche en Sciences de la Sante (IRSS), Burkina Faso; Centre de Support en Sante Internationale (CSSI), Chad; The University Gamal Abdel Nasser, Conakry (GANU), Guinea; Malaria Research Training Centre (MRTC), Mali; Epicentre, Niger; Le Centre de Recherche Médicale et Sanitaire (CERMES), Niger; Epidemiological Resources and Investigation Consultancy (ERIC), Nigeria; Jedima International Health Consult Ltd. (JIHCL), Nigeria; Medical Research Council-The Gambia (MRC), The Gambia; Universite Cheikh Anta Diop, Senegal; TDR, Switzerland; WHO, Switzerland
Funding Body: UNITAID through Malaria Consortium and Catholic Relief Services
Seasonal Malaria Chemoprevention (SMC) is the administration of effective antimalarial drugs once a month to children under 5 years of age, during the rainy season, in order to prevent malaria. The ACCESS-SMC project was undertaken to improve availability of SMC drugs, to expand access to SMC, and evaluate the effectiveness of the strategy at scale, in seven high-burden countries: Burkina Faso, Chad, The Gambia, Guinea, Mali, Niger and Nigeria.
SMC was implemented in a phased manner in 2015 and 2016 to a total of about 7million children. Children were surveyed each year to verify uptake, national pharmacovigilance systems in each country were strengthened to monitor adverse drug reactions, case control studies were used to estimate the protective efficacy of monthly treatments, the frequencies of molecular markers of drug resistance were measured in large-scale surveys, and the impact in terms of the reduction in the number outpatient cases of malaria and the number of children dying from malaria in hospital, was assessed using cases reported in national health management information systems and by collecting individual patient data before and after SMC introduction from health facilities. Costs of SMC were estimated in each country and cost effectiveness and net cost savings were estimated.
The study showed that high, equitable coverage was achieved overall, in 2016 90% of eligible children received at least one SMC treatment and 54% received all four treatments, but coverage varied with some countries performing better than others. Each monthly treatment provided a high degree of protection for 4 weeks. Molecular monitoring showed that drug resistant infections are very uncommon, but the situation may change and needs careful monitoring. Monitoring of safety was strengthened during the project but these efforts need to be sustained. Serious side effects were rare. SMC cost $3.8 per child per year, and was highly cost-effective. There was a substantial reduction in the number of malaria cases, and the number of deaths from malaria in hospital, when SMC was introduced. In two countries with a DHIS2 (District Health Information System 2) databases established prior to SMC scale-up, Burkina Faso and The Gambia, introduction of SMC was associated with a reduction in the number of malaria deaths in hospital during the high transmission period, of 44% (Burkina Faso) and 57% (The Gambia). Despite the complexity of delivery, SMC has proved highly effective.
The mechanisms underlying the production of natural mosquito repellents by human beings
Location of study: LSHTM, UK; MRC, The Gambia
LSHTM Investigators: James Logan, Umberto D’Alessandro, Julien Martinez, Catherine Oke, Thomas Ant, Elizabeth Pretorius
External collaborators: John Armour, (University of Nottingham, UK); Steven Lindsay, (Durham University, UK); John Pickett, (Rothamsted Research, UK)
Funding Body: MRC
A recent study demonstrated that human genetics plays an important role in determining our attractiveness to mosquitoes. We are measuring the attractiveness of identical and non-identical twins to malaria-transmitting mosquitoes, and using genome sequence data to identify the genes involved. If the genes can be identified, we can use this information to develop novel methods to protect against malaria.
We have collected odour compounds from the skin of 90 pairs of identical and non-identical twins using two methods: 1) asking the twins to wear a nylon sock for 24 hours, and 2) using a technique called air entrainment by which skin volatiles are captured and dissolved in a solvent. Levels of attractiveness of the different socks are quantified in wind tunnel behavioural experiments, in which female mosquitoes are presented with the choice of flying towards a sock worn by a participant or an unworn sock. Gas-chromatography analysis is being used in parallel to identify and quantify individual volatile compounds from the solvent solutions, to determine which compounds are associated with attractiveness.
In collaboration with Nottingham University, we plan to use existing genomic data from the twins to perform an association analysis and identify genes linked to the production of volatile compounds and attractiveness to mosquitoes. With the MRC Unit The Gambia we are conducting a similar study throughout 2019, measuring attractiveness in a human population naturally exposed to malaria. The aim here is to investigate whether genes play a role in the modulation of attractiveness, caused by infection.
Program for Resistance, Immunology, Surveillance, and Modelling of malaria (PRISM2): Insecticide Resistance Project
Location of study: Uganda
LSHTM Investigators: Henry Ddumba Mawejje, Sarah Staedke, Jo Lines
External collaborators: Martin Donnelly, (Liverpool School of Tropical Medicine, UK); Sam Nsobya and Moses Kamya, (Makerere University/Infectious Diseases Research Collaboration Uganda); Grant Dorsey, (University of California, San Francisco, US)
Funding Body: National Institutes of Health (NIH) - National Institute of Allergy and Infectious Diseases (NIAID)
Insecticide resistance remains a major threat to malaria control. This project will assess the status and intensity of insecticide resistance, evaluate its interaction with vector control and malaria transmission, and identify novel resistance-associated genes in local malaria vector populations.
This study will answer fundamental questions about insecticide resistance, mosquito survival and sporozoite infection using single nucleotide polymorphism (SNP) associations. The study will involve repeated cross-sectional monitoring of measures of phenotypic and genotypic insecticide resistance in different mosquito populations throughout Uganda. Mosquitoes will be collected from the catchment area of the Uganda Malaria Surveillance Project (UMSP) Malaria Reference Centers (MRCs), and other sites of national interest. The specific objectives of the project include (1) to evaluate the association between SNPs that confer insecticide resistance (Cyp4j5, Coeae1d, kdr L1014S, kdr L1014F, & Ace - 1 119S) and mosquito survival in insecticide exposed An. gambiae s.l. adults; (2) to evaluate the association between resistance SNPs and sporozoite infection in wild caught An. gambiae s.s.; and (3) to evaluate the association between vector control interventions and the prevalence of SNPs that confer insecticide resistance in An. gambiae s.l. mosquitoes across time and space. Initially, we will screen for resistanceassociated polymorphisms in An. gambiae and An. arabiensis, as these are likely the primary vectors in most study locations, but this may change over time. The results of this project will provide an improved understanding of the scope and intensity of insecticide resistance in Uganda.