ESORT (Emergency Surgery – Or noT) is a two-year NIHR-funded project aiming to determine the effectiveness and cost-effectiveness of emergency surgery for patients with common acute conditions presenting as emergency hospital admissions.
ESORT will apply an instrument-variable design to answer the study objectives. Data will be Hospital Episode Statistics on acute conditions collected from hospitals in England for 2009-2020
The Emergency Surgery – OR noT (ESORT) study aims to determine the effectiveness and cost-effectiveness of emergency surgery for patients with common acute conditions presenting as emergency admissions to NHS hospitals.
Many patients with common acute conditions such as appendicitis are admitted to hospital in an emergency. Some will have surgery but others will not. For most patients, it is unclear whether the benefits of emergency surgery are greater than the risks.
This uncertainty means there are large differences in the way patients are treated across England. We know that over half (56%) of patients admitted to NHS hospitals in England with common acute conditions had emergency surgery. Almost half did not. Even for patients with similar conditions, there were big differences in the proportion who received emergency surgery in different hospitals.
Our study will help us understand the benefits, risks and costs involved in emergency surgery. We want to ensure that unnecessary surgery does not happen - but that surgery does happen for people who need it.
- ESORT C-19
-
The ESORT team have received funding from the Health Foundation to research the effectiveness and cost-effectiveness of surgery during the COVID-19 pandemic, in order to inform decision-making during the recovery period.
Overview
Following the COVID-19 outbreak, planned surgery in the NHS was cancelled. As a result, NHS waiting lists are now approaching 10 million patients. The NHS urgently needs evidence to inform which patients should be prioritised for emergency surgery. Information for patients on the risks and outcomes of emergency surgery must recognise the impact of COVID-19.
This research study will assess the effect of COVID-19 on access to emergency surgery for different patient groups, and will report on the effectiveness and cost-effectiveness of emergency surgery versus conservative management following the COVID-19 outbreak. Evidence-based recommendations, which can be quickly incorporated into NHS guidelines, will be developed on which patients should be prioritised for emergency surgery in the COVID-19 recovery phase.
Team
The ESORT team have been joined by Susan Moug and Ravi Vohra (Advisory Group members on the NIHR-funded ESORT project) as co-applicants on ESORT-C19.
Funder
ESORT-C19 is funded as part of the Health Foundation’s COVID-19 research programme.
More details about the study can be found on the Health Foundation website.
PPI
The PPI panel on the NIHR-funder ESORT project were invited to support ESORT-C19 through workshops held in May 2022. Read the Microsoft Sway information that was sent to panelists.
Read notes from the meeting.
- ESORT Vascular
-
The ESORT Vascular study expands upon the ESORT studies and looks to determine the effectiveness and cost-effectiveness of emergency surgery for patients with common vascular conditions.
Many people require major surgery for conditions affecting blood vessels to prevent strokes, limb amputation and death. These operations are high risk and costly and require careful planning to achieve good patient outcomes.
Sometimes, specialists recommend that patients plan for elective surgery at a later date, so that they can have medications, invasive heart treatments or exercise classes to help them recover from surgery. However, delaying surgery can be dangerous, as major blood vessel disease can deteriorate rapidly. Patients may also feel anxious about waiting for surgery. So, for most patients, it is unclear whether it is better to proceed to more urgent surgery or have elective surgery at a later date.
The guidelines about how long patients should wait before having surgery are based on weak evidence, and so, there is wide variation in the average time to surgery across the NHS. For example, for patients the average time from seeing a surgeon to undergoing surgery, ranges across NHS hospitals from 4 to 42 days for surgery than can prevent a stroke.
The overarching aim is to estimate the effectiveness and cost-effectiveness of urgent versus elective surgery for common, vascular conditions, recognising COVID-19’s impact on vascular surgery provision.
The specific objectives are to evaluate the:
- Clinical and cost-effectiveness of urgent versus elective surgery for common vascular conditions.
- Clinical and cost-effectiveness of urgent versus elective surgery for specific patient subgroups, including diagnostic subcategories and patient characteristics.
- Provide recommendations about which patient subgroups should have ‘urgent’ versus ‘delayed’ surgery, and define subpopulations and comparison groups for a RCT.
Methods
We will use routinely collected information about patients who have had major blood vessel surgery. We will apply modern statistical methods to these previously collected data, to assess which groups of patients (e.g. age groups or types of blood vessel disease) would benefit more from either urgent or elective surgery.
Results
Study results will be discussed with PPI and clinical panels, presented at conferences and published in peer-review journals. We will use our close links with key organisations to include the study findings in clinical guidelines. We will share our findings with patients and the public through the study website, newsletters and social media.
PPI
Two experienced patient co-applicants, and PPI panellists from the NIHR ARC North Thames PPI panel have informed our study design, by emphasising the importance of the research, and of reporting outcomes (e.g. days alive and out of hospital), that matter to patients. The study will be supported by the Bristol Vascular Patient Advisory Group, and the Vascular Society of Great Britain and Northern Ireland special interest groups, that include ex-patients with these conditions. We will hold two virtual PPI panels who will advise on how best to explain the study and its finding to patient groups.
Privacy notice
Read our privacy notice.
This section provides more detailed information about the study design for researchers. More information is available on the NIHR website and the study protocol (pdf).
Research question
How effective and cost-effective is emergency surgery for patients with common acute conditions presenting as emergency hospital admissions?
There is little evidence to inform whether patients with acute conditions who present as emergency hospital admissions should have emergency surgery, or alternative non-operative care. The aim of this 24-month study is to evaluate the effectiveness and cost-effectiveness of emergency surgery for common acute conditions (listed below), that present as emergency admissions to NHS hospitals.
The 'intervention' strategy is emergency surgery within the index hospital episode, and the 'comparator' strategy is non-operative care including: 'medical management', a 'non-surgical' procedure, and the possibility of subsequent planned (elective) surgery.
The conditions that ESORT will be studying are:
- appendicitis
- diverticulitis
- cholelithiasis
- intestinal obstruction
- abdominal wall hernia
Objectives
The specific objectives of the ESORT study are to evaluate the:
- effectiveness of emergency surgery versus non-operative care for common acute conditions presenting as emergency admissions across broad ICD-10 categories.
- relative cost-effectiveness of emergency surgery versus non-operative care across broad ICD-10 categories.
- clinical and cost-effectiveness of operative versus non-operative care for specific patient subgroups, including diagnostic subcategories and patient characteristics.
Methods
The study will apply an instrumental variable (IV) design that can provide accurate estimates of treatment effectiveness, even when there are unmeasured prognostic differences between the comparison groups. This study will extend an IV design previously developed in the United States by co-applicant Dr. Luke Keele, that uses surgeon's tendency to operate as an instrument to evaluate emergency surgery versus non-operative strategies. We will include five conditions with well-defined intervention and comparator strategies where there is clinical uncertainty, and wide variation in rates of emergency surgery across the NHS.
For the effectiveness analysis, the main outcomes include: mortality up to one year after the index emergency admission, hospital re-admissions, and days alive and out of hospital up to 30 days after the index admission. The cost-effectiveness analyses (CEA) will use mortality and resource use data from HES and the Office for National Statistics, and quality of life data extracted from the literature. We will report results according to pre-specified subgroups including patient demographics, comorbidities and route of admission.
Data
We will use Hospital Episode Statistics (HES) data on acute conditions collected from hospitals in England for 2009-2020. See privacy section for further information.
Ethics
The study received approval from the LSHTM ethics committee in April 2020.
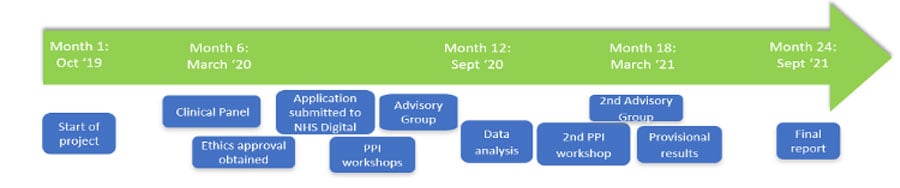
Timeline
ESORT is led by a team of health economists at the London School of Hygiene and Tropical Medicine in collaboration with clinicians and other experts. An Advisory Group, with an independent chair, oversees the study. PPI and Clinical Panels provide specialist guidance to the study.
Claire Snowdon
Paul Charlton
What is PPI?
PPI means ‘Patient and Public Involvement’ and is a core component of our research. It helps us to ensure that our researchers are asking the right questions and using the right methods, so that our studies will provide answers that will make a real difference to people’s lives.
If you would like to know more about PPI, this video explains more about the value of involving patients and the public in research. It is made by our funders, National Institute for Health Research (NIHR). You can read more about patient and public involvement in research on the NIHR/INVOLVE website.
PPI in the ESORT study
PPI in the ESORT study is managed by our PPI co-applicants, Paul Charlton and Claire Snowdon.
In the ESORT study, we want to have a clearer understanding of what is important for patients, their families and the public in general when someone arrives at a hospital in an emergency. We had some thoughts at the start of the project about what outcomes are important, such as simply surviving the emergency, or how long someone stays in hospital, or if they are readmitted. We wanted to discuss these with members of the public and former patients. We also wanted to discuss how to judge how people feel about their quality of life after experiencing going into hospital as an emergency.
These views and insights help us to understand more about the real-life experiences behind the information that we are collecting from hospital medical records.
The PPI Panel
We hosted two sets of virtual panels – at the design stage of the study in July 2020 and in September 2021, once we had our study findings. At these panels, we spoke to members of the public and former patients about our study.
We have invited participants who:
- Had experienced coming into hospital as an emergency due to appendicitis, diverticulitis or gallstones
- are a family member/carer of someone who has had this experience
- have not had this experience but would like to help the study
We include here a number of materials from our panels.
From the July 2020 design panels, you can see the materials that we shared with our participants in advance on Microsoft Sway – there is the general information pack, plus our patient stories. You can also see a note of our meetings, for further information about what we discussed.
From the September 2021 panels, you can see the Microsoft Sway our participants used and read a note of our meetings. At the September panels, we also shared the Plain English summary of our study with our participants.
Accessible Information Project
Alongside the ESORT PPI work, Paul has worked with a graphic designer at Thinklusive, adults with learning disabilities and/or autism and the Leicester Centre for Black and Ethnic Minority Health on an accessible information project to create information about how our study uses large personal health information data sets. They developed an ‘easy-read’ overview of the study approach and the updated version with the study results.
Alongside the ESORT PPI work, we have also started a parallel accessible information project:
Paul will work with a graphic designer, adults with learning disability and/or autism and the Leicester Centre for Black and Ethnic Minority Health. They will co-create information about how our study uses large personal health information data sets.
A set of three slides, explaining what we propose to do is available.
This study is funded by the National Institute for Health Research Health Services and Delivery Research (project number 18/02/25).
You can find out more information about the study on the NIHR website:
Please note: the views and opinions expressed on this website are those of the research team and do not necessarily reflect those of the Health Services and Delivery Research Programme, NIHR, NHS or the Department of Health.
ESORT Clinical Panel – A report summarising ESORT’s clinical panel, which met virtually in March 2020 with subsequent work then undertaken over email. The panel had three main purposes: (1) to refine the inclusion and exclusion criteria for defining the study population; (2) to refine the list of procedures within the definition of ‘emergency surgery’; and (3) to define ‘the most appropriate’ time window that constitutes ‘emergency surgery’.
ESORT Analysis Plan – This document details the proposed presentation and analysis for the main paper(s) reporting results from objective 1 of the Emergency Surgery Or noT (the ESORT study) to evaluate the effectiveness of emergency surgery (ES) versus non-emergency surgery (NES) strategies for five common acute conditions: acute appendicitis, cholelithiasis, diverticular disease, abdominal wall hernia or intestinal obstruction that present as emergency admissions to NHS hospitals.
ESORT Submitted Paper – This paper, and its supplementary material, describe the groups of patients whose hospital records have been analysed in the ESORT study. The paper examines associations between patient characteristics and receipt of ES. It found older age is associated with a lower likelihood of ES for some conditions. The paper also examines variation in ES rates between NHS Trusts after adjustment for patient characteristics and found greatest variation in cholelithiasis. This article has been accepted for publication in BJS Open, published by Oxford University Press.
Anaesthesia: The final paper from the ESORT study: Effectiveness of emergency surgery for five common acute conditions: an instrumental variable analysis of a national routine database. - Listen to the podcast of the 3 key authors discussing the study. Read the editorial on our ESORT Study.
LSHTM handles any data used in the ESORT study in accordance with data protection laws and its own internal policies and procedures. Read LSHTM’s data protection policy.
The main way in which the ESORT study uses data is by receiving information from NHS Digital, the nation’s provider of information and data in health and social care. We will receive it under a data sharing agreement. There is more information about how we use data in this way in our ESORT privacy notice.
Additionally, we will receive information about people who participate in the Patient and Public aspect of our study. Their information is governed by LSHTM’s Research Participant Privacy notice.
Andrew Hutchings presented to the ASGBI conference, which was held virtually in May 2021.
Andrew presented the early findings from the ESORT study, which show that, for patients admitted to hospital with common acute conditions, rates of emergency surgery were lower for older patients, after adjustment for other patient characteristics.
Access the abstract.
The ESORT team has completed the first Clinical Panel for the project. As noted in a previous update, the Panel was originally intended to be face-to-face in London, but due to the COVID-19 pandemic, the first stage of the panel took place over the Zoom platform with subsequent work undertaken via email.
The team has now finalised the report from this panel, which it will use to further the design of the project. The report is available in the Publications section of this website.
The team convened its first Advisory Group meeting on 15 July 2020, chaired by Professor Iain Anderson MBE, a consultant at Salford Royal NHS Foundation Trust.
The Advisory Group comprises a number of independent clinicians and academics, plus the Public & Patient co-applicant (Paul Charlton). The Group has a key oversight role, ensuring that the project is well-managed and on course to fulfil its objectives.
A further Advisory Group meeting will take place later on in the project, once the team has preliminary results from its data analysis.
On 6 and 9 July, the ESORT team hosted two virtual Patient and Public Involvement (PPI) panels. These panels sought the views of members of the public and individuals who had experience of the conditions being studied in ESORT.
Due to the COVID-19 pandemic, the panels took place over the Zoom platform, with the pre-reading made available via Microsoft Sway.
Panelists discussed outcomes of treatment, as well as quality of life issues. Topics of discussion included pain-management and the psychological impact of treatment, as well as the potential impact of COVID-19 on decisions about care.
Panelists were asked to join a subsequent panel to discuss the results of the ESORT study. The ESORT team is immensely grateful to the PPI panelists for lending their time to support the project.
Patient and Public Involvement (PPI) is a core component of our research. We are in the process of hosting two virtual workshops, on 6 July and 9 July 2020, at which we will speak to members of the public and former patients about our study. We will seek their views on issues such as outcomes of surgery and quality of life. There is more information about PPI on the website of our funder, NIHR.
The project team was pleased to obtain ethics approval for the ESORT project in March 2020. The approval was granted by the LSHTM Research Ethics Committee, which reviews all research undertaken by LSHTM staff or students. Read more information on ethics at LSHTM.
As part of the ESORT study, we have convened a Clinical Panel, which seeks specialist views on key elements of the study, such as what constitutes emergency surgery. This had been intended to be a face-to-face meeting in London, bringing together a number of clinicians, mainly surgeons. Due to COVID-19, the first stage of this panel took place online on Friday 27 March 2020, with further work undertaken offline. The team is in the process of finalising the report of this panel, which it will use to further the design of the project.