Intestinal schistosomiasis is a complex parasitic disease that affects millions of children in worldwide. With only one drug to treat it (praziquantel), there is still no optimal dosing for children under 5 years of age.
Intestinal schistosomiasis is a complex parasitic disease that affects millions of children worldwide. With only one drug to treat it (praziquantel), there is still no optimal dosing for children under 5 years of age.
The PIP trial (Praziquantel in Preschoolers) is a five year randomised controlled trial investigating different doses and treatment intervals of praziquantel given to children under five years of age. It will also explore the role of intestinal pathology on the pharmacokinetics and pharmacodynamics of praziquantel and its role in drug efficacy.
Collaborating partners
| Brown University-Center for International Health Research | LSHTM-MRC Unit | Vector Control Division Uganda Ministry of Health |
University of Liverpool |
Funder
The PIP Trial is funded by the National Institutes of Health in the USA. It brings together LSHTM, Brown University, the LSHTM MRC unit in Uganda and the Vector Control Division from the Ministry of Health in Uganda.
Jennifer Friedman
Principal investigator (US)
Patrice Mawa
Principal investigator (Uganda)
Andrew Edielu
Our wonderful team presented baseline data at the American Society of Tropical Medicine and Hygiene (ASTMH) in Seattle.
Initial baseline results of the PIP trial were presented at the American Society of Tropical Medicine and Hygiene in Seattle.
Sophie Pach presented the baseline ultrasound findings of the PIP trial. Results highlighted incipient fibrosis in preschool children.
Suzanne presented baseline results on morbidity markers in preschool children with intestinal schistosomiasis. Anaemia was found to be associated with high intensity S.mansoni infection.
Andrew Edielu presented baseline results on proxy markers of gut inflammation and blood in stool- both biomarkers were associated with S.mansoni infection.
Congratulations to Andrew Edielu, Sophie Pach and Suzanne Colt.
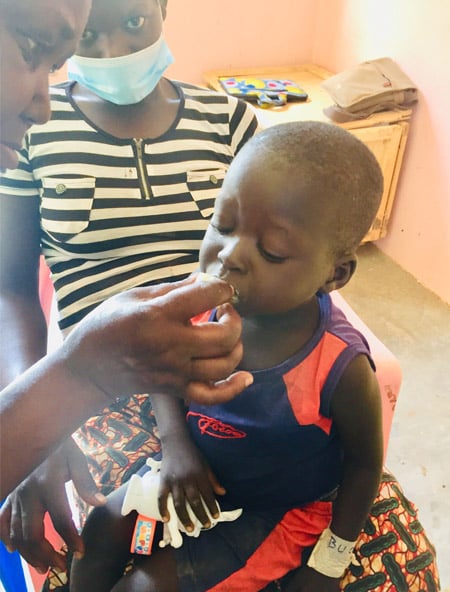
We have started recruiting at our research camp in Lake Albert on April 16, 2021. Excellent team efforts!