Thanks to an investment from the Bill and Melinda Gates Foundation, a team of researchers led by a central group at LSHTM are conducting the first multi-national prospective observational study to better understand the mortality burden due to AMR in bloodstream infections in sub-Saharan Africa.
Mbira instrument
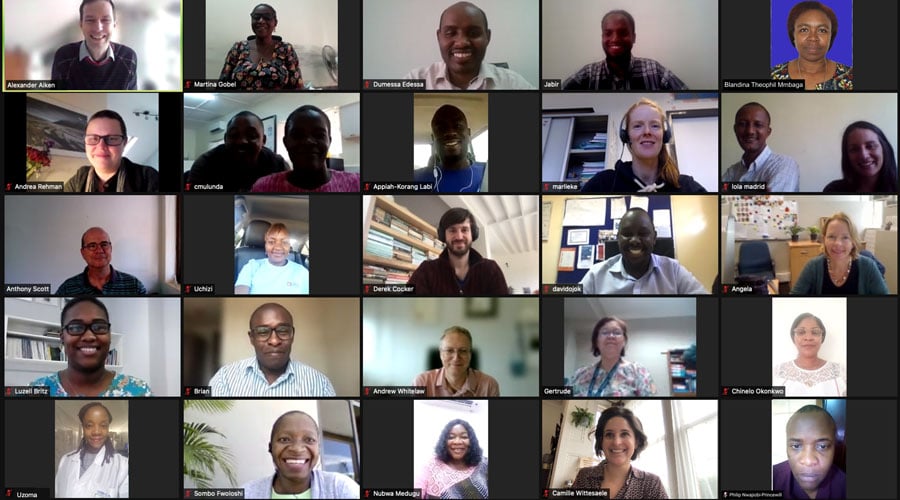
The MBIRA study team is comprised of dozens of researchers working across 9 different countries in sub-Saharan Africa.
Antimicrobial resistance (AMR) threatens the effective prevention and treatment of a range of infections caused by bacteria, viruses, parasites, and fungi globally. Whilst AMR is a global issue, it has major implications for African countries where severe bacterial infections are common but access to effective antibiotic treatment remains limited. To date, we lack reliable estimates of the impact of AMR in low and middle income country (LMIC) settings, which limits our capacity to address the increasing threat.
Thanks to an investment from the Bill and Melinda Gates Foundation, a team of researchers across nine African countries, led by PI Alex Aiken at LSHTM, are conducting the first multi-national prospective observational study to better understand the mortality burden due to AMR in bloodstream infections in sub-Saharan Africa.
This substantial, multi-site study will provide generalizable, credible estimates to policy makers and clinicians to drive local, national and global AMR policy. With appropriate estimates of the burden of AMR, countries will be able to make more informed decisions on resource allocation, policy-making, and committing to new research.
The MBIRA project is focussed on bloodstream infections (=bacteraemia) caused by Gram-negative enteric bacteria (Enterobacteria, such as E. coli and Klebsiella pneumoniae) and includes patients of all age groups, from neonates to adults. The aim is to gather data across a range of hospitals in sub-Saharan Africa to inform larger global burden of disease analyses.
Across our partner research sites, data collection will take place in local hospitals from November 2020 and continue through to early 2022. We anticipate the first findings to be analysed by mid-2022.
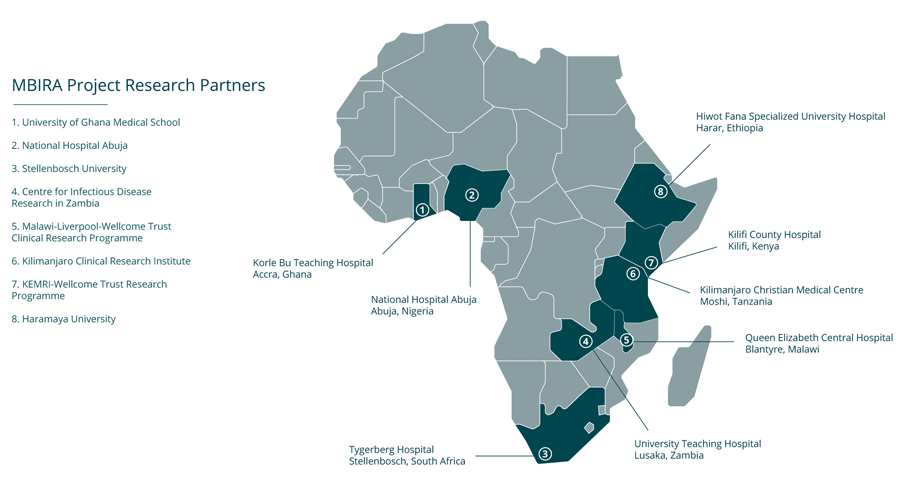
The MBIRA study team is comprised of dozens of researchers working across 8 different countries in sub-Saharan Africa, including staff based at the following research institutions:
- Centre for Infectious Disease Research in Zambia (Lusaka, Zambia)
- Haramaya University (Harar, Ethiopia)
- Kilimanjaro Clinical Research Institute (Moshi, Tanzania)
- KEMRI-Wellcome Trust Research Programme (Kilifi, Kenya)
- Malawi-Liverpool-Wellcome Trust Clinical Research Programme (Blantyre, Malawi)
- National Hospital Abuja (Abuja, Nigeria)
- Stellenbosch University (Stellenbosch, South Africa)
- University of Ghana Medical School (Accra, Ghana)
The study aims to include patients from hospitals affiliated with each of our research partners above, all collecting the same types of data prospectively. We hope to recruit a total of approximately 1,200 patients with bacteraemia across all sites in the study, matching to approximately 2,400 non-infected patients in the same hospitals.
“The MBIRA study is timely, giving the rising cases of antimicrobial resistance (AMR) worldwide. Although the negative impacts of AMR are well documented, the true burden is not well studied, particularly in the low- and middle-income countries like Nigeria. The study outcome is expected to fill this yawning gap in knowledge and will provide data to inform policies and planning. The hospital stands to benefit from the study as we hope to obtain a mirror of the current state of antibiotic resistance. The study also provides an opportunity for capacity building for our staff taking part in it, in addition to the networking opportunity it has afforded.”
- Dr Kenneth C. Iregbu, MBBS, MSc, MPH, MD, FMCPath (Nig), FCPath (ECSA), FWACP-Lab Med, FRCPath (UK); Consultant Clinical Microbiologist, National Hospital Abuja & Senior Lecturer, Department of Medical Microbiology, College of Health Sciences, University of Abuja, Nigeria
“The Tygerberg Hospital/Stellenbosch University site is a 1324-bed teaching hospital in Cape Town, South Africa and participated in the MBIRA I study. Gram-negative bloodstream infections (BSI), both community- and hospital-acquired are leading infective causes of mortality in South Africa. Klebsiella pneumoniae is the dominant pathogen and is frequently antibiotic-resistant, with about 2/3rds of isolates producing extended-spectrum B-lactamases (ESBL) and about 5% exhibiting carbapenem-resistance. The MBIRA II study will provide crucial first estimates of the impact of antibiotic resistance on patient outcomes of Gram-negative BSI in African hospitals. These data will inform local policies guiding empiric antibiotic selection for suspected BSI and generate valuable estimates for future studies aiming to reduce infection-related morbidity and mortality in Africa.”
- Prof Angela Dramowski, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
“The impact of antimicrobial resistance (AMR) on health systems and populations are estimated to be high but have not been adequately documented. In low middle income countries such as Ghana, the impact of AMR is expected to be high for reasons such as inaccessibility to effective antimicrobials. We are happy to part of this multi-national MBIRA study because it will help accurately document the impact of AMR not only in Ghana but Africa. It is our hope that findings from this study will serve as evidence for national and international bodies during planning, resource allocation and advocacy for activities geared towards AMR fight.”
- Dr. Appiah-Korang Labi, MBChB, MGCP, PhD; Clinical Tutor, Department of Medical Microbiology, University of Ghana, Medical School
Mortality associated with third-generation cephalosporin resistance in Enterobacteriaceae bloodstream infections at one South African hospital
Angela Dramowskia, Alexander M. Aiken, Andrea M. Rehman, Yolandi Snyman, Sandra Reuter, Hajo Grundmann, J. Anthony G Scott, Marlieke E.A. de Kraker, Andrew Whitelaw.
Read the publication.
MBIRA Publication in JAC – Antimicrobial Resistance
Mortality attributable to third-generation cephalosporin resistance in Gram-negative bloodstream infections in African hospitals: a multi-site retrospective study.
Angela Dramowski, Gerald Ong’ayo, Andrea M Rehman, Andrew Whitelaw, Appiah-Korang Labi, Noah Obeng-Nkrumah, Awa Ndir, Marcelyn T Magwenzi, Kenneth Onyedibe, Martin Wolkewitz, Marlieke E A de Kraker, J Anthony G Scott, Alexander M Aiken, MBIRA study collaborators, Volume 3, Issue 1, March 2021
Access a PDF of the open access article.
Antimicrobial Resistance Centre Seminar
Measuring impacts of Gram-negative bacteraemia in Sub-Saharan Africa: Challenges for a new prospective study.
Presented by Alex Aiken, 6 October 2020
WHO GLASS Methodology
GLASS method for estimating attributable mortality of antimicrobial resistant bloodstream infections.
World Health Organisation, 2020
Access a PDF of the WHO resource.
Statistical analysis plan
This statistical analysis plan is a document describing the planned statistical analyses for the MBIRA study, written in advance of inspection of the final study dataset (Feb 2022).
Training manual
This training manual is the main document used for training sites participating in the MBIRA study – this may be of interest to anyone planning a similar study in the future.
Antibiotic-appropriateness guide
This antibiotic-appropriateness guide is a training document used to describe in detail the MBIRA study approaches to classifying antibiotic use as “appropriate” (or concordant) with laboratory testing results. It may be of interest to anyone planning a similar future study.