Timing is everything: Evidenced research on vaccine schedules is critical in saving lives
London School of Hygiene & Tropical Medicine https://lshtm.ac.uk/themes/custom/lshtm/images/lshtm-logo-black.png Tuesday 6 June 2023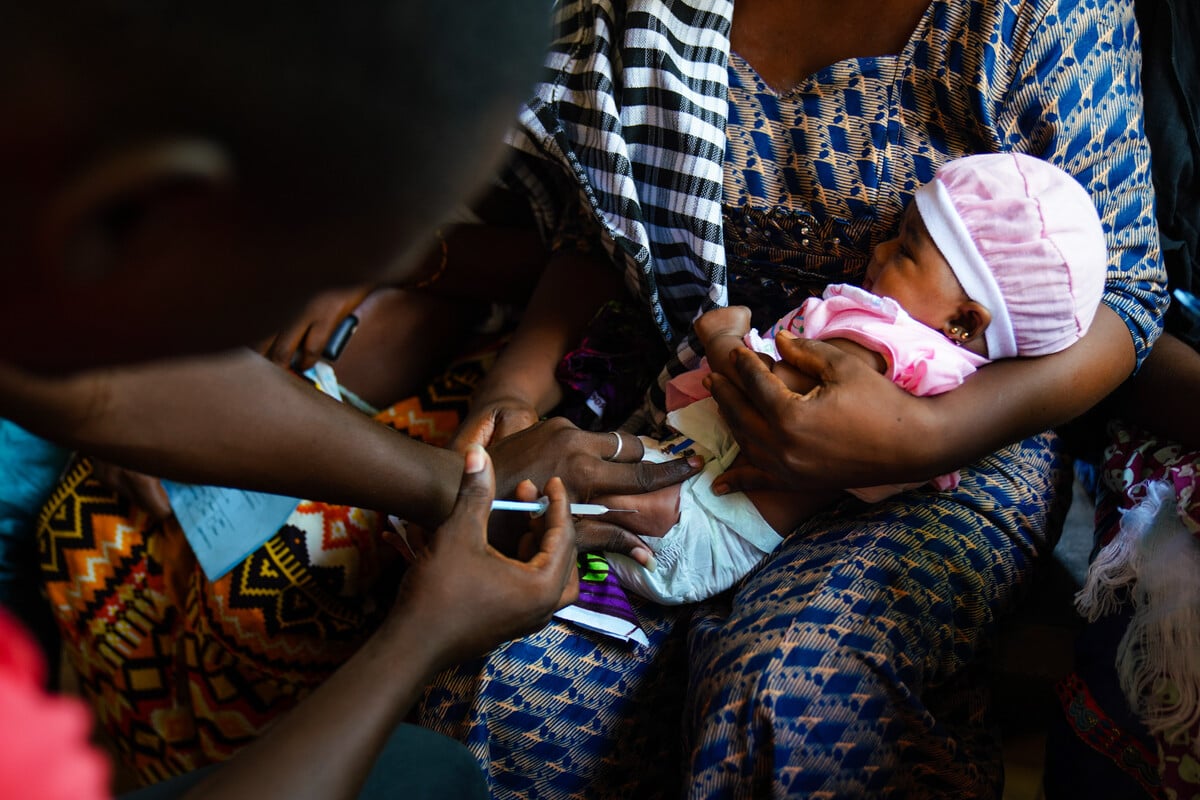
As clinical researchers, we are motivated by knowing that the type of work and research we do can ultimately make a positive impact on our global health. We hope to contribute to scientific knowledge that will inform policy decisions on the introduction and use of vaccines. We also hope to improve their accessibility and affordability. This is crucial for reducing the burden of vaccine-preventable diseases like pneumonia in low- and middle-income countries, and ensuring that vulnerable populations are not left behind.
Streptococcus pneumoniae (pneumococcus) is a bacterium with over 90 different strains, known as serotypes. About 50% of people infected with it are asymptomatic carriers, however it can cause both non-invasive diseases, such as ear and sinus infections, and invasive diseases.
For instance, invasive pneumococcal disease occurs when the bacteria cross into sterile areas such as blood or cerebrospinal fluid. This causes diseases like pneumonia, meningitis, and sepsis. Pneumococcal disease is a major cause of mortality and morbidity worldwide, particularly among children less than five years in low- and middle-income countries. Great strides have been made with the introduction of vaccines which have led to a 65% reduction globally in related deaths among under-fives between 1990 and 2019. However, even with these improvements, there are still over 370,000 deaths annually for these vulnerable children worldwide.
Pivotal research undertaken at the Medical Research Council Unit The Gambia at the London School of Hygiene & Tropical Medicine has demonstrated the efficacy of pneumococcal vaccines in preventing childhood illness in low- and middle-income countries.
Our studies conducted between 2015 and 2018 demonstrated the safety and immunogenicity of a more affordable vaccine that targets the 10 main pneumococcal bacterial strains responsible for the majority of related diseases in these settings.
This ‘10-valent pneumococcal conjugate vaccine’ (PCV) - with the pharmaceutical name Pneumosil – was developed by the Serum Institute of India in collaboration with PATH. It is one of only three PCVs currently prequalified by the World Health Organization, and approved for use in a schedule of three primary doses in early infancy (3+0) or three doses plus a booster (3+1).
In this study, we conducted a follow up Phase 3 trial in The Gambia alongside the Serum Institute and PATH, to investigate the safety and immunogenicity of Pneumosil with two doses in early infancy, and a booster dose at 9-18 months. These 2+1 schedules are thought to have the advantage of conferring longer lasting protection compared to 3+0.
We enrolled 660 healthy infants aged 6-8 weeks who had never received a PCV, and were then randomly assigned to receive one of three vaccines: Pneumosil or one of the other two pneumococcal conjugate vaccines prequalified by the WHO; the 10-valent Synflorix (GlaxoSmithKline, UK); or the 13-valent Prevenar 13 (Pfizer, USA; given routinely in The Gambia). Ultimately, we wanted to see the immune response to each vaccine four weeks after the booster.
Our recent results showed that Pneumosil was safe when administered in a 2+1 schedule, and likely to provide similar protection as the other existing vaccines. There were no notable vaccine-related safety concerns.
Based on this research, the WHO has approved an update to the Pneumosil package insert to indicate it is suitable for administration in a 2+1 schedule.
This means there is expanded access to effective and less costly pneumococcal vaccination in countries where a 2+1 schedule has already been introduced, or is under consideration – as is the case across most of Europe, north Africa, and South America. Pneumosil is available at $2 per dose which is 30% less than Gavi’s price for similar PCV’s and significantly less than non-Gavi prices.
Vaccine affordability has significant impact on public health outcomes, and Pneumosil increases accessibility to countries with limited resources and can also be used more effectively, providing longer-lasting protection. This will reduce the burden of illness and death, alongside the healthcare costs of treating the diseases.
This research highlights the critical role that evidence plays in informing policies that positively impact public health practices. It underscores the potential for innovative and efficient strategies to expand access to life-saving vaccines, especially in low and middle-income countries.
This is a significant breakthrough in the fight against pneumococcal disease and it is crucial that investment in research and development is continued to optimise the use of existing vaccines, in order to maximize their benefits, reduce the burden of disease - and ultimately save lives.
LSHTM's short courses provide opportunities to study specialised topics across a broad range of public and global health fields. From AMR to vaccines, travel medicine to clinical trials, and modelling to malaria, refresh your skills and join one of our short courses today.