OPSIN: Optimising serological surveillance for malaria in Indonesia
Location of study: Indonesia
LSHTM Investigators: Henry Surendra, Chris Drakeley, Jackie Cook
External collaborators: Supargiyono & Riris Ahmad (Centre for Tropical Medicine, Faculty of Medicine, Public Health and Nursing, Gadjah Mada University, Indonesia)
Funding Body: Indonesia Endowment Fund for Education
This project explores the feasibility of optimising use of serological surveillance for estimating the magnitude and heterogeneity of malaria transmission in a pre-elimination setting in Indonesia. We integrate novel serological and geographical information system techniques with the existing public health surveillance system to better understand malaria transmission dynamics in this setting.
We found that rolling health facility-based cross-sectional surveys involving all facility attendees and their companions provide an alternative approach for quickly obtaining parasitological, serological, geolocation and epidemiological data. Simple addition of the use of mobile technology-based participatory mapping approaches into the surveys could provide attractive approach for remotely collecting accurate fine-scale geolocation data to study spatial pattern of disease in low resource areas without formal addresses and poor internet connection.
Although the parasiteprevalence was very low (5/10,000), analysis of serological samples against a panel of novel antigens could facilitate the measurement of recent and historical exposure to P. falciparum, P. vivax and P. knowlesi as well as the characterisation of the spatial patterns and risk factors of malaria exposure in a pre-elimination setting in Indonesia. In conclusion, use of serological surveillance could be optimised by utilising existing surveillance system to monitor disease transmission dynamics for control and elimination programmes planning. However, further implementation research is needed to enable integration of these methods with existing surveillance systems.
Bias in routinely collected Plasmodium falciparum malaria surveillance data due to asymptomatic infections according to transmission intensity: A pooled analysis of paired health system and community cross-sectional survey data
Location of study: Worldwide (Brazil, Cambodia, Ethiopia, The Gambia, Haiti, Kenya, Malaysia, Myanmar, Peru, Philippines, Tanzania, Zambia)
LSHTM Investigators: Gillian Stresman, Nuno Sepulveda, Chris Drakeley, Kimberly Fornace, Shunmay Yeung, Lynn Grignard (LSHTM, UK); Umberto D’Alessandro, Julia Mwesigwa, Jane Achan (MRC Unit The Gambia)
External collaborators: Katherine Battle, Ewan Cameron and Peter Gething, (Malaria Atlas Project, University of Oxford, UK); Andre Siqueira, (Fundacao Oswaldo Cruz, Rio de Janeiro, Brazil); Emilie Poithin and Joanna Gallay, (Swiss Tropical and Public Health Institute, Basel, Switzerland); Siv Sovannaroth, (NMCP, Phnom Penh, Cambodia); Jennifer Stevenson and Antonio Quispe, (Johns Hopkins Bloomberg School of Public Health, Baltimore, US); Teun Bousema and Fitsum Tadesse, (Radboud University Medical Center, Nijmegen, The Netherlands); Effie Espino, Joy Lorenzo and Malou Macalinao, (Research Institute for Tropical Medicine, Manilla, Philippines); Jordi Landier, Gilles Delmas and Francois Nosten, (MAHIDOL Oxford Tropical Medicine Research Unit, Bangkok, Thailand); Jacklin Mosha, (NIMR, Mwanza, Tanzania); Thomas Eisele, (Center for Applied Malaria Research and Evaluation, Tulane University, US); John Miller and Daniel Bridges, (PATH, Lusaka, Zambia); Michelle Chang and Karen Hamre, (Centers for Disease Control and Prevention, Atlanta, US); Alyssa Young, (Clinton Health Access Initiative, Port-au-Prince, Haiti); Jean Frantz Lemoine, (Programme National de Controle de la Malaria, Port-auPrince, Haiti)
Funding Body: Wellcome Trust
Health systems data is the foundation information that malaria programs use to estimate malaria burden and inform control/elimination programming. However, the proportion of infected individuals that do not seek care, and thus not included in these statistics, can be significant. This work seeks to understand how well health systems data can mirror the total burden of infection, and how this changes according to transmission intensity. The aim was to determine the relationship between the proportion of all infections in the community that are identified within the health system and transmission intensity, as a proxy for levels of protective immunity in the community.
Paired community and health system data, concordant in both time and space, were collected from 435 clusters in 12 countries. The proportion of infections detected in the health system was estimated according to the number of infections detected at the facility using a binomial distribution and the total number of infections in the community according to a hypergeometric distribution. Uncertainty was determined by bootstrapping. The proportion detected was modelled according to transmission intensity, measured by overall population PCR prevalence.
Preliminary results indicate the proportion of infections detected by the health system starts to increase when overall malaria prevalence in the community is less than ~10%. Facilities detect up to 100% of infections in non-African settings when the probability of seeking care and bednet use are adjusted for. In African settings, the proportion of infections detected gradually increases linearly. These findings suggest that once transmission is sufficiently low, health systems can be relied upon to detect most malaria infections in a community.
Malaria Zero: The Alliance for A Malaria-Free Haiti. Convenience sampling vs. household survey gold standard: assessment of spatial bias in predicted malaria exposure
Location of study: Haiti
LSHTM Investigators: Chris Drakeley, Gillian Stresman, Lotus van den Hoogen, Kevin Tetteh, Nuno Sépulveda
External collaborators: Katherine Battle and Ewan Cameron, (Malaria Atlas Project, University of Oxford, UK); Thomas Eisele, Ruth Ashton and Thomas Druetz, (Center for Applied Malaria Research and Evaluation, Tulane University, US); Michelle Chang and Karen Hamre, (Centers for Disease Control and Prevention, Atlanta, US); Bernadette Fouche, (CDC Foundation, Port-au-Prince, Haiti); Jean Frantz Lemoine, (Programme National de Controle de la Malaria, Port-au-Prince, Haiti)
Funding Body: Bill & Melinda Gates Foundation
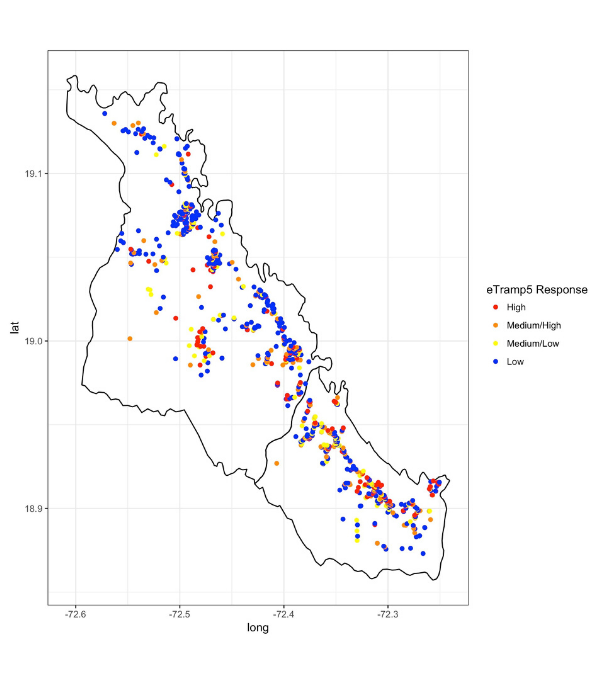
Maps showing the distribution of malaria are becoming an important tool for control and elimination activities. Here, we are determining if maps generated from data collected using convenient sampling methods can identify the same areas of high malaria exposure compared to the more accurate but labour intensive community-based data collection.
Convenience sampling in easy access groups (EAG) to measure malaria burden provides an attractive alternative to household (HH) surveys. Malaria seroprevalence estimates generated by sampling school-children as well as all health facility attendees have both been shown to provide reasonable estimates of malaria transmission in the community. Here, we assess the degree of spatial bias when generating maps of malaria seroprevalence according to the EAG sample compared to the gold standard HH survey conducted in the Artibonite Valley, Haiti. For the EAG study, 2126 children in 22 schools and 2108 individuals attending 9 health facilities were sampled with spatial coordinates of the household available for 1092 participants. EAG seroprevalence were compared to fitted estimates according to a geostatistical model according to data from a HH survey of 21620 individuals residing in 6847 households.
Preliminary results suggest that estimates from the EAG study overall underestimated seroprevalence, with a smaller degree of bias in the health facility sample. However, the maps generated from the EAG data were able to identify similar areas of high burden as the HH survey in areas where the EAG and HH survey data overlapped spatially.
Antimalarial antibody detection assays: in search of a standardised tool to detect the absence of transmission
Location of study: Bataan, The Philippines; Praia, Cape Verde & LSHTM, UK
LSHTM Investigators: Lotus van den Hoogen, Nuno Sépulveda, Gillian Stresman, Kimberley Fornace, Kevin Tetteh & Chris Drakeley
External collaborators: Paolo Bareng, Ralph Reyes, Jennifer Luchavez, Malou Macalinao and Fe E. Espino (Research Institute for Tropical Medicine, Department of Health, the Philippines); Julio Rodrigues and Joana Alves (National Institute of Public Health, Cabo Verde); Alan Kitchen, (NHS Blood and Transplant, UK); Peter Chiodini, (Hospital for Tropical Diseases, UK)
Funding Body: Bill & Melinda Gates Foundation
The lack of antibody responses to malaria in young children signifies the absence of exposure to infections. As such, antibody detection may help in declaring an area malaria-free. At present there are no standardised assays to measure malaria antibodies for epidemiological use. There are several commercial assays but these have been developed to screen blood donations prior to transfusion. We aimed to evaluate the performance of commercially available assays for epidemiological use.
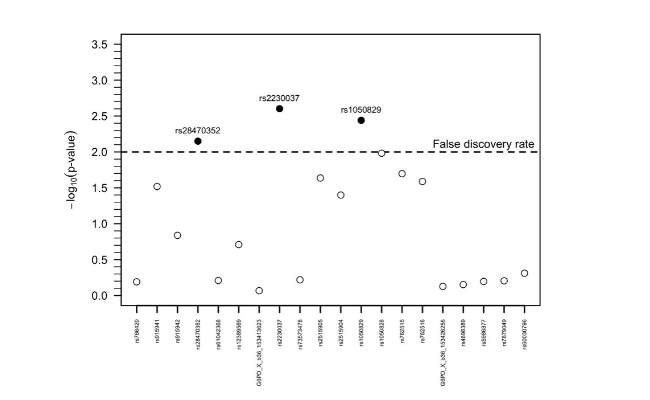
We compared five commercially available ELISA antibody detection assays for malaria. For Phase I, we assessed the ease-of-use, cost, sample volume needed and specificity (using malaria-naïve; n=223 as well as toxoplasma positive, malaria-naïve samples; n=191). Based on these results, one assay was taken forward for epidemiological evaluation. For Phase II, samples from Bataan, the Philippines were assayed (n=1824; an area without clinically reported cases at local health facilities since the early 90s) as well as Praia, Cape Verde (n=1396; unstable, low transmission over the past decades). Preliminary results from Bataan show a change-point in the force of infection (SCR: seroconversion rate), which overlapped with the recorded drop in malaria cases. When a more stringent threshold for seropositivity was used, only four children under the age of 16 were seropositive while the recent SCR was 0.0001 (95% confidence interval, 0.0000-0.0004), Figure 1.
Whether the four detected seropositive children indicate responses from asymptomatic infections, outliers of seronegative responses, or cross-reactive responses to antigens from other pathogens is unknown. These results are promising in the development of standardised serological assays as adjunct measures to confirm claims of cessation of malaria transmission at a sub-regional level.
Fever case management in urban slums in Uganda
Location of study: Kampala, Uganda
LSHTM Investigators: Sian Clarke , Eleanor Hutchinson, Daniel Chandramohan
External collaborators: Anthony Mbonye (Ministry of Health, Uganda); Phyllis Awor, Miriam Kayendeke, Elizeus Rutebemberwa & Esther Buregyeya (Makerere University School of Public Health, Uganda); Pascal Magnussen & Kristian Hansen (University of Copenhagen, Denmark)
Funding Body: MRC/ESRC/DfID/Wellcome Trust: (Development Grant, Health Systems Research)
Malaria, pneumonia and diarrhoea are major causes of death in African children, yet if diagnosis and treatment are available most of these deaths can be prevented. Integrated community case management (iCCM) by community health workers (CHWs) can improve access to treatment in rural areas. However use of CHWs in urban settings is low, and other ways to provide health care for the urban poor are needed. Growing cities are characterised by a vibrant private sector of formal and informal providers, who potentially could be trained to deliver iCCM.
Snowball sampling was used to identify all treatment providers in two high-density urban slums in Kampala, Uganda. Data on treatment practices and quality of care were collected through interviews with private sector providers, audit of drug stocks, and observations of consultations for childhood fever. We also conducted focus group discussions with health providers and community members.
Our findings revealed the vast number and diversity of private providers that operate in urban settings, even in low-income areas – with 72 private clinics, drug shops, and pharmacies identified in the two sites. Almost all were located within 30 minutes’ walk of a public health centre. Over 70% of premises were licensed and staffed by a qualified health worker (nurse or medical doctor). All reported selling antimalarials, and over 70% reported selling amoxicillin and other antibiotics. Treatment for malaria and diarrhoea were consistent with national guidelines, but the diagnosis and treatment of pneumonia was poor, across all types of providers. In brief, though premises were usually operated by a qualified health worker, there remains ample scope for treatment services to be improved.
ACT treatment of uncomplicated Plasmodium falciparum infections, and co-infecting P. knowlesi, P. malariae and P. vivax, in North Sumatera
Location of study: North Sumatera, Indonesia
LSHTM Investigators: Inke Lubis, Sarah Staedke, Colin Sutherland
External collaborators: Hendri Wijaya, Munar Lubis, Chairuddin Lubis (Department of Paediatrics, University of Sumatera Utara, Indonesia)
Funding Body: Directorate General of Higher Education, Indonesia
Growing evidence of drug resistant malaria in the Greater Mekong Region has led to concerns that this problem may spread to neighbouring countries of SE Asia. We set out to test the effectiveness of current malaria drugs in Nth Sumatera Province, Indonesia, as data from this region has been unavailable.
Plasmodium falciparum with reduced susceptibility to the artemisinin family of drugs, and harbouring variant alleles of pfk13, are present in the SE Asian region. We conducted an openlabel, randomised comparison of dihydroartemisinin-piperaquine and artemether-lumefantrine for the treatment of uncomplicated falciparum malaria, with or without other co-infecting Plasmodium, in North Sumatera province, Indonesia. 153 and 149 individuals with microscopy-confirmed P. falciparum malaria were randomised into the two treatment arms, respectively. In the evaluable intention to treat population (n=144, 146 respectively) drug efficacy at day 42 was 84.0% for dihydroartemisinin-piperaquine and 90.4% for artemether-lumefantrine ( P=0.09). The PCR-corrected efficacy at day 42 was 99.3% and 100% ( P=0.31), respectively. Post hoc species-specific PCR analysis at all time-points identified a significant proportion of parasite-negative individuals among the randomised population, indicating poor specificity of microscopic diagnosis at enrolment.
Nine cases of microscopically-detected parasitaemia in follow-up were confirmed by PCR as P. malariae, P. knowlesi, P. vivax or a mixture of species. qPCR estimates of parasiteclearance up to 72h post-treatment indicated that, in our study area, P. falciparum is susceptible to artemisinin. However, P. falciparum DNA was detected at day 28 or day 42 in 35.1% of evaluable individuals, suggesting a failure of prophylactic protection from lumefantrine and piperaquine. Pre-treatment analysis found the SVMNT pfcrt haplotype at codons 72-76 dominant in these parasite populations; the pfk13 propeller domain variant T474A, of unknown phenotypic significance, was identified in 3 individuals. We found strong evidence of post-treatment selection for the Pfmdr1 NF haplotype at codons 86 and 184 in both drug groups.
K13-independent mechanisms of reduced artemisinin susceptibility in Plasmodium falciparum
Location of study: LSHTM
LSHTM Investigators: Ryan Henrici, Donelly van Schwalkwyk, Colin Sutherland
Funding Body: UK Foreign and Commonwealth Office through the Marshall Scholarship Programme
The effectiveness of artemisinin, a current malaria drug, has been falling in SE Asia over the last decade. This is linked to mutations in the gene encoding the parasite protein K13. We have been examining other ways that parasites can become less susceptible to artemisinin, as these may be relevant in Africa.
We have been examining the effects of mutations in two P. falciparum genes, pfap2mu (Figure) and pfubp1, on artemisinin susceptibility in vitro. We have used CRISPR-Cas9 gene editing to introduce mutations of interest, and have succeeded in generating parasite lines with a significantly enhanced ability to survive a 4h pulse of 700 nM dihydroartemisinin. We have also shown that transient exposure to low termperature, and the use of agents that inhibit intracellular trafficking produce a similar affect. In each case, the parasites did not carry mutations in the gene encoding the K13 protein.
Interestingly, none of these genes or other factors, including K13, can generate artemisinin resistance beyond the ring-stage of parasite development; all parasite stages are fully susceptible from 8-10h post-invasion onwards. Reduced parasite susceptibility is thus a partial adaptation to the brevity of artemisinin exposure in vivo, giving a proportion of early parasites an improved chance of surviving in the treated host. Work continues to unravel the biological basis of this interesting phenomenon.
Quality of antimalarial medicines sold in Bioko Island, Equatorial Guinea
Location of study: Bioko Island, Equatorial Guinea
LSHTM Investigators: Harparkash Kaur, Elizabeth Louise Allan, Ibrahim Mamadu, Zoe Hall
External collaborators: Michael D Green and Isabel Swamidoss, (Division of Parasitic Diseases and Malaria, US Centers for Disease Control and Prevention, Atlanta, US); Facundo M Fernández, Prabha Dwivedi and Maria Julia Culzoni, (Georgia Institute of Technology, School of Chemistry and Biochemistry, Atlanta, US); Feliciano Monti, Dianna Hergott and Guillermo Garcia, (Bioko Island Malaria Control Project, Equatorial Guinea)
Funding Body: Ministry of Health and Social Welfare Malabo, Equatorial Guinea (through Medical Care Development International Bioko Island Malaria Control Project)
Access to good quality, efficacious medicines is essential to treat malaria. Poor-quality medicines, including falsified, substandard, and degraded medicines, pose serious health concerns in malaria endemic countries, with potential lead to drug resistance and kill patients. Systematic assessment of drug quality will provide evidence for Ministries of Health to take action.
The first line medicines to treat malaria, namely the artemisinincontaining antimalarials (ACAs) were purchased using three sampling approaches on Bioko Island, Equatorial Guinea. Samples (677 of from 278 outlets) were purchased as follows: convenience survey using mystery client (n=16 outlets, 31 samples), full island-wide survey using mystery client (n=174 outlets, 368 samples) and randomised survey using an overt sampling approach (n=88 outlets, 278 samples).
The stated active pharmaceutical ingredients (SAPIs) were assessed using high-performance liquid chromatography and confirmed by mass spectrometry at three independent laboratories. Content analysis showed 91.0% of ACAs were of acceptable quality, 1.6% were substandard and 7.4% falsified. No degraded medicines were detected. The prevalence of medicines without the SAPIs was higher for ACAs purchased in the convenience survey compared to the estimates obtained using the full islandwide survey-mystery client and randomised overt sampling approaches. Comparable results were obtained for full-island survey mystery client and randomised overt.
Falsified ACAs were found on Bioko Island, with the prevalence ranging between 6.1-16.1%, depending on the sampling method used. These findings underscore the vital need for national authorities to track the scale of ineffective medicines that jeopardise treatment of life-threatening diseases, and value of a representative sampling approach to obtain/measure the true prevalence of poor quality medicines in a given geographical region.
Bead based assays to simultaneously detect multiple human inherited blood disorders associated with malaria
Location of study: Burkina Faso and The Gambia
LSHTM Investigators: Lynn Grignard, Catherine Mair, Bronner P. Gonçalves, Taane G. Clark, Susana Campino, Chris Drakeley
External collaborators: Jonathan Curry and Laleta Mahey, (LGC genomics, UK); Kjerstin H.W. Lanke, Guido J.H. Bastiaens and Teun Bousema, (Radboud University, The Netherlands); Alfred B. Tiono, Sam A. Coulibaly, Alphonse Ouédraogo, Edith C. Bougouma, Guillaume Sanou, Issa Nébié and Sodiomon B. Sirima, (CNRFP, Burkina Faso); Joseph Okebe, Muna Affara and Umberto d’Alessandro, (MRC The Gambia)
Funding Body: The Bill and Melinda Gates Foundation
We aimed to develop a new, cheap and quick method to simultaneously detect human genetic changes in a single reaction. The genetic changes of interest are associated with the severity of malaria or the treatment of the disease.
Glucose-6-phosphate dehydrogenase deficiency (G6PDd), haemoglobin C (HbC) and S (HbS) are inherited blood disorders (IBD) common in populations in malaria endemic areas. All are associated to some degree with protection against clinical malaria whilst additionally G6PDd is associated with haemolysis following treatment with 8-aminoquinolines. Current methods in epidemiological studies are time consuming and relatively low throughput.
Our new assay detects G6PD SNPs and common haemoglobin mutations (HbS and HbC). In a pilot study, seventy-five samples from Burkina Faso (n=75/78, 96.2%) and fifty-eight samples from The Gambia (n=58/61, 95.1%) had a G6PD and a HBB genotype successfully assigned by the assay. Flow cytometry data further supported the concordance between G6PD enzyme activity and genotype. The bead based assay compares well to alternative measures of genotyping and phenotyping for G6PD. The screening is high throughput, and easily standardised to include more targets.
LINK (LSHTM-INFORM-NMCP-Knowledge) Data for malaria decision-making
Location of study: Demographic Republic of the Congo (DRC), Ghana, Kenya, Malawi, Mali, Mozambique, Nigeria, Republic of South Sudan, Republic of Sudan, Senegal, Sierra Leone, Tanzania and Uganda
LSHTM Investigators: Caroline Lynch, David Schellenberg
External collaborators: Robert Snow (KEMRI-Wellcome Trust Research Programme, Nairobi, Kenya); WHO Regional Office for Africa, Brazzaville, DRC
Funding Body: UK Department for International Development (DFID)
The LINK project aimed to; work with National Malaria Programmes (NMPs) in 13 countries to generate detailed epidemiological profiles of malaria risk and intervention coverage, work with WHO AFRO to assist national and regional decision-makers improve evidence-based decision making; and improve capacity of NMPs to update profiles in future.
LINK produced 24 epidemiological profiles for the highest burden malaria countries in sub-Saharan Africa. Multiple requests for reprofiling were made by National Malaria Programmes (NMPs), indicating the value placed on LINK profiling and engagement. Our evaluation found that LINK epidemiological profiles were used for strategic and operational decisions by NMPs including; tailoring interventions to epidemiological strata, prioritising resource allocation, identifying where interventions should be implemented, and critically analysing trends over time to determine potential reasons for increases in risk associated with gaps in coverage.
We identified several challenges to using data for decisionmaking. These included; fragmented data making it difficult for NMPs to have a comprehensive view of programme issues, lack of availability of data in a timely enough way for strategic decision-making, a lack of understanding and subsequent mistrust of modelled estimates of malaria risk, challenges in interpreting discordant data from different sources, and tension between the global call for universal coverage and local needs to target resources because of insufficient funding. There is an emerging culture of data use at national level, which needs to be strengthened as NMPs begin to think about the most effective mix of interventions for different epidemiological strata.