Vaccine Centre COVID-19 response
Many members of the LSHTM Vaccine Centre are involved in the global response to the COVID-19 pandemic. Staff and students are involved in many different types of research including modelling the pandemic, social science research on social responses, and involvement in lab work and trials. In addition, many members of the Centre are clinicians involved in the front line response.
The Vaccine Centre will continue to be active in the coming months by organising public and member engagement activities and sharing up-to-date and evidence-based resources.
The articles and resources shared on this page will be on the COVID-19 response, evolution of the pandemic, and the progress towards a COVID-19 vaccine. The Centre is also keen to highlight and share research on the collateral or indirect impacts of the pandemic, such as research that shows what the diversion of resources away from routine vaccination programmes could have around the world.
Resources
Current COVID-19 vaccine candidates:
-
Vaccine development pipeline for COVID-19
-
Dr Edward Parker and Professor Beate Kampmann from the Vaccine Centre have developed a tracker tool for vaccines currently in the pipeline against COVID-19. This will be updated on a regular basis: https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/
From the beginning of September 2020 the VaC tracker includes a 'living review' summarising results from published trial reports as they become available: : https://www.lshtm.ac.uk/newsevents/expert-opinion/keeping-track-development-covid-19-vaccine
- WHO list of vaccine candidates
-
The World Health Organization curated a list of current COVID-19 vaccine candidates at the end of March 2020 which can be accessed here: www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1
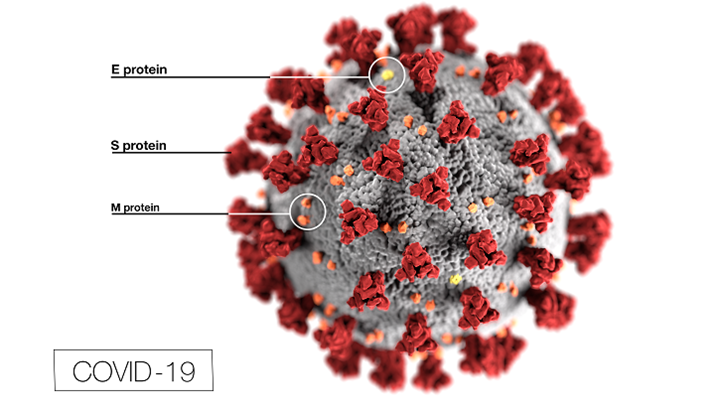
Irrespective of the strategy, the majority of vaccines in current development use the Spike (S) glycoprotein of SARS-CoV-2 as the antigen. Either the full length protein or fragments of it (especially the RBD, ACE2 Receptor Binding Domain).
COVID vaccine delivery:
- COVAX: towards global equitable access to COVID-19 vaccines
-
COVAX is a new initiative convened by Gavi, CEPI and the WHO. COVAX will act as a platform to support research, development and manufacturing of a wide range of COVID-19 vaccine candidates, and negotiate their pricing. This global collaboration now involves 170 countries - representing 70 per cent of the global population. COVAX will help to make available the vaccine to low-income countries involved in the initiative.
Vaccine trials and therapeutics:
-
Pandemic vaccine development challenges
-
The Coalition for Epidemic Preparedness Innovations (CEPI) affiliated authors have published a useful commentary on pandemic vaccine development challenges and concerns in the New England Journal of Medicine: www.nejm.org/doi/full/10.1056/NEJMp2005630
- Global regulator issue guidelines for COVID-19 vaccine trials
-
Global regulators published a report on the 24th of March presenting outcomes of a workshop on COVID-19 vaccine development, convened under the umbrella of the International Coalition of Medicines Regulatory Authorities (ICMRA). The meeting report provides an overview of regulatory considerations related to COVID-19 vaccine development and data required for regulatory decision-making.
-
How vaccine trials speed up during a pandemic
-
Gavi, The Vaccine Alliance has published a page on how the normal stages of vaccine development has changed in the context of a pandemic situation. The rapidly developed Ebola vaccine is a recent example of a sped up process: www.gavi.org/vaccineswork/how-clinical-vaccine-trials-are-speeding-pandemic
Other LSHTM COVID-19 resources:
- Sustaining routine immunisation during the COVID-19 pandemic
-
The Centre for Mathematical Modelling of Infectious Diseases (CMMID) at LSHTM have published work that weighs up the health benefits of continued routine infant immunisation delivery in Africa against the risk of acquiring coronavirus infections through visiting vaccination services. Access the report here: https://cmmid.github.io/topics/covid19/control-measures/EPI-suspension.html
LSHTM Viral
Tune into LSHTMs podcast series on the science behind outbreaks and COVID-19, new episodes posted regularly: https://anchor.fm/lshtm
LSHTM's COVID-19 response
Stay up to date with all LSHTM activities on COVID-19 on the main website here: www.lshtm.ac.uk/research/research-action/covid-19